| CPC A61K 47/6455 (2017.08) [A01K 67/027 (2013.01); A61K 38/08 (2013.01); A61K 38/44 (2013.01); A61K 47/549 (2017.08); A61K 47/58 (2017.08); A61K 48/0041 (2013.01); C08F 293/005 (2013.01); C12N 15/113 (2013.01); C12N 15/87 (2013.01); A01K 2207/05 (2013.01); C12N 2310/14 (2013.01); C12N 2310/351 (2013.01); C12N 2320/32 (2013.01); C12Y 113/12007 (2013.01)] | 18 Claims |
|
1. A block copolymer of the formula I
T1-L1-[A]x-[B]y—Z I
wherein
T1 is a first targeting moiety;
L1 is absent or a linking moiety;
A is a first block that is a polymer formed from monomers comprising formula A2 or a random copolymer formed from monomers comprising formulae A2 and A3; A2, A4 and A5; A2 and A5; or A4 and A5;
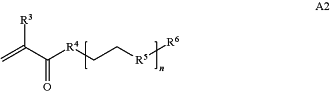 wherein n is 1-120, R3 is H or C1-C6 alkyl, R4 is S, O, NH or N(C1-C6 alkyl), R5 is O or S and R6 is H, C1-C6 alkyl, C1-C6 alkyl-NH2, C1-C6 alkyl-NH(C1-C6 alkyl), or C1-C6 alkyl-N(C1-C6 alkyl)2;
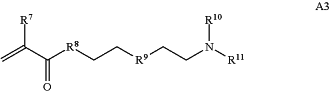 wherein R7 and R10 are independently H or C1-C6 alkyl, R8 is S, O, NH or N(C1-C6 alkyl), and R9 is O or S and R11 is an amine protecting group;
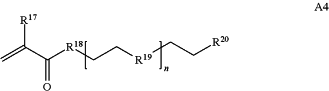 wherein n is 1-230, R17 is H or C1-C6 alkyl, R18 is O, S, NH or N(C1-C6 alkyl), R19 is O or S, and R20 is OH, NH, H, T2, or C1-C6 alkyl, where T2 is a second targeting moiety;
 wherein R21 is H or C1-C6 alkyl, R22 is O, NH or N(C1-C6 alkyl), R23 is H, aryl, arylhalide, alkyl, or alkyl alcohol;
B is a second block that is a random copolymer formed from monomers consisting of formulae B1, B2, and B3
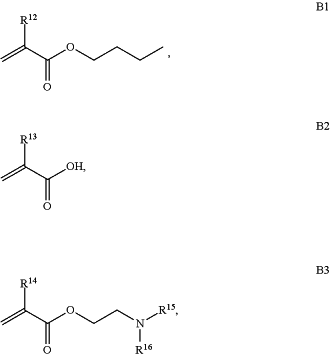 wherein R12, R13, R14, R15, and R16 are independently H or C1-C6 alkyl;
x is 2-20 kDa;
y is 2-20 kDa;
the ratio of x to y is from 2:1 to 1:4; and
Z is H, SH, C(CH3)2CN,
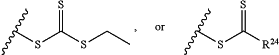 wherein R24 is S—(C1-C12 alkyl), aryl, arylhalide, O—(C1-C12 alkyl), or NR25R26 wherein R25 and R26 are independently H, alkyl, aryl, or heteroaryl; and
 designates a point of attachment. designates a point of attachment. |
|
7. A method for the intracellular delivery of an oligonucleotide comprising:
a) contacting a cell with a block copolymer of the formula I
T1-L1-[A]x-[B]y—Z I
wherein
T1 is a first targeting moiety;
L1 is absent or a linking moiety;
A is a first block that is a polymer formed from monomers comprising formula A2 or a random copolymer formed from monomers comprising formulae A2 and A3; A2, A4 and A5; A2 and A5; or A4 and A5;
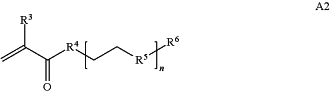 wherein n is 1-120, R3 is H or C1-C6 alkyl, R4 is S, O, NH or N(C1-C6 alkyl), R5 is O or S and R6 is H, C1-C6 alkyl, C1-C6 alkyl-NH2, C1-C6 alkyl-NH(C1-C6 alkyl), or C1-C6 alkyl-N(C1-C6 alkyl)2;
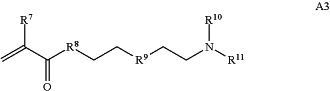 wherein R7 and R10 are independently H or C1-C6 alkyl, R8 is S, O, NH or N(C1-C6 alkyl), and R9 is O or S and R11 is an amine protecting group;
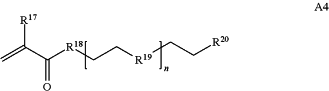 wherein n is 1-230, R17 is H or C1-C6 alkyl, R18 is O, S, NH or N(C1-C6 alkyl), R19 is O or S, and R20 is OH, NH, H, T2, or C1-C6 alkyl, where T2 is a second targeting moiety;
 wherein R21 is H or C1-C6 alkyl, R22 is O, NH or N(C1-C6 alkyl), R23 is H, aryl, arylhalide, alkyl, or alkyl alcohol;
B is a second block that is a random copolymer formed from monomers consisting of formulae B1, B2, and B3
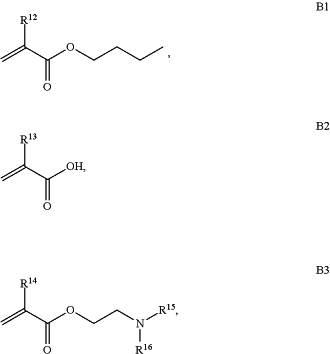 wherein R12, R13, R14, R15, and R16 are independently H or C1-C6 alkyl,
x is 2-20 kDa;
y is 2-20 kDa;
the ratio of x to y is from 2:1 to 1:4; and
Z is H, SH, C(CH3)2CN,
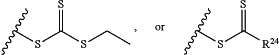 wherein R24 is S—(C1-C12 alkyl), aryl, arylhalide, O—(C1-C12 alkyl), or NR25R26 wherein R25 and R26 are independently H, alkyl, aryl, or heteroaryl; and
 designates a point of attachment; with a cell and designates a point of attachment; with a cell andb) contacting the cell with the oligonucleotide, wherein the oligonucleotide is delivered to the cytosol of the cell.
|
|
13. A method for treating a disease or condition associated with defective gene expression and/or activity in a subject, the method comprising administering to a subject in need thereof
a) a block copolymer of the formula I
T1-L1-[A]x-[B]y—Z I
wherein
T1 is a first targeting moiety;
L1 is absent or a linking moiety;
A is a first block that is a polymer formed from monomers comprising formula A2 or a random copolymer formed from monomers comprising formulae A2 and A3; A2, A4 and A5; A2 and A5; or A4 and A5;
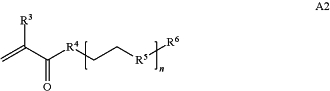 wherein n is 1-120, R3 is H or C1-C6 alkyl, R4 is S, O, NH or N(C1-C6 alkyl), R5 is O or S and R6 is H, C1-C6 alkyl, C1-C6 alkyl-NH2, C1-C6 alkyl-NH(C1-C6 alkyl), or C1-C6 alkyl-N(C1-C6 alkyl)2,
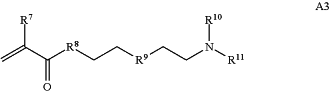 wherein R7 and R10 are independently H or C1-C6 alkyl, R8 is S, O, NH or N(C1-C6 alkyl), and R9 is O or S and R11 is an amine protecting group;
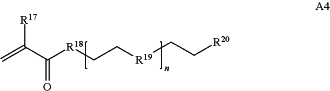 wherein n is 1-230, R17 is H or C1-C6 alkyl, R18 is O, S, NH or N(C1-C6 alkyl), R19 is O or S, and R20 is OH, NH, H, T2, or C1-C6 alkyl, where T2 is a second targeting moiety;
 wherein R21 is H or C1-C6 alkyl, R22 is O, NH or N(C1-C6 alkyl), R23 is H, aryl, arylhalide, alkyl, or alkyl alcohol;
B is a second block that is a random copolymer formed from monomers consisting of formulae B1, B2, and B3
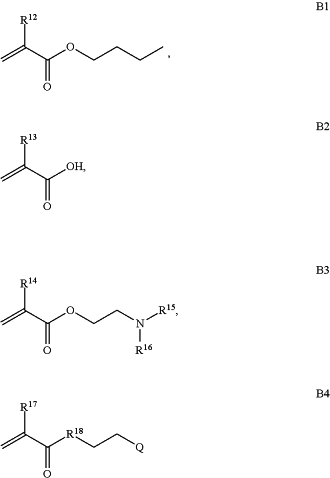 wherein R12, R13, R14, R15, and R16 are independently H or C1-C6 alkyl,
x is 2-20 kDa;
y is 2-20 kDa;
the ratio of x to y is from 2:1 to 1:4; and
Z is H, SH, C(CH3)2CN,
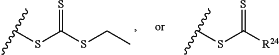 wherein R24 is S—(C1-C12 alkyl), aryl, arylhalide, O—(C1-C12 alkyl), or NR25R26 wherein R25 and R26 are independently H, alkyl, aryl, or heteroaryl; and
 designates a point of attachment; designates a point of attachment;and
b) a therapeutically effective amount of an oligonucleotide.
|