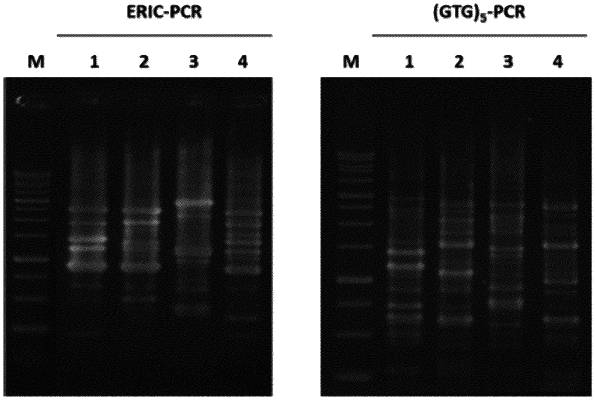
|
1. A method for (i) treating or preventing inflammatory bowel syndrome a subject in need thereof, (ii) treating or preventing anxiety, inflammation, and/or HPA axis dysfunction in a subject suffering from maternal separation (MS) and/or (iii) treating or preventing sarcopenia in a subject in need thereof, comprising administering the isolated and purified lactic acid bacteria Lactobacillus paracasei PS23 (PS23), deposited with Deutsche Sammlung von Mikroorganismen und Zellkulturen under Budapest Treaty and was given the accession number DSM 32322 or a composition comprising PS23 to the subject, wherein the PS23 is administered in an amount ranging from about 1×107 to about 1×1013 cells/day of heat-killed PS23 cells or about 1×107 to about 1×1013 CFU/day of live PS23 cells.
|