| CPC A61B 5/1468 (2013.01) [A61L 2/03 (2013.01); A61N 1/18 (2013.01); A61L 27/28 (2013.01)] | 16 Claims |
|
1. A method for treating a metallic surface of an implantable device in order to eradicate infectious bacteria, the method comprising:
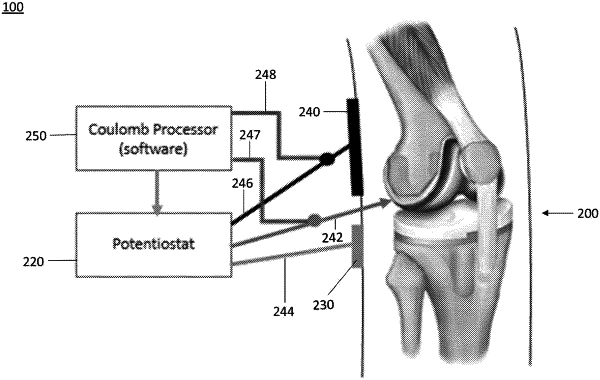
a) enabling a stimulation device of a treatment system capable of applying a DC voltage sufficient to disrupt the bacteria from the metallic surface of the implantable device, the DC voltage being applied between a pair of electrodes, and in which one of the electrodes is the implantable device;
b) using a processor of the treatment system, measuring an accumulated charge transfer to the metallic surface of the implantable device over time, the accumulated charge transfer being measured in coulombs;
c) comparing the measured accumulated charge transfer to a predetermined charge transfer threshold level stored by the processor; and
d) continuing to apply the DC voltage until the measured accumulated charge transfer exceeds the predetermined charge transfer threshold level.
|