| CPC A61B 17/12036 (2013.01) [A61B 17/12109 (2013.01); A61B 17/12145 (2013.01); A61F 2/848 (2013.01); A61F 2/915 (2013.01); A61F 2002/068 (2013.01); A61F 2230/005 (2013.01); A61F 2250/0039 (2013.01)] | 15 Claims |
|
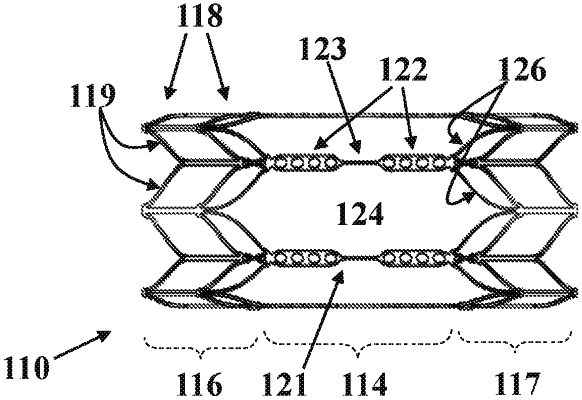
1. An implant for gradually restricting vascular blood flow, comprising:
an elongated implant body comprising a flow restricting portion enclosing a variable minimal internal diameter; at least one first holding member configured for restraining the implant body to maintain the variable minimal internal diameter in a first minimal internal diameter, the at least one first holding member is configured to physically yield voluntarily after a first predetermined average duration of being continuously subjected to internal human body conditions, thereby releasing and allowing the implant body to elastically deform voluntarily such that the variable minimal internal diameter changes to a second minimal internal diameter, smaller than the first minimal internal diameter; and
at least one second holding member configured for restraining the implant body to maintain the variable minimal internal diameter in the second minimal internal diameter, after the first holding member physically yields, the second holding member is configured to physically yield voluntarily following a second predetermined average duration, greater than the first average duration, of being continuously subjected to human body conditions, thereby releasing and allowing the implant body to elastically deform voluntarily such that the variable minimal internal diameter changes to a third minimal internal diameter, smaller than the second minimal internal diameter.
|