| CPC G16H 40/40 (2018.01) [G06F 16/2455 (2019.01); G06N 5/02 (2013.01); G06N 20/00 (2019.01); G06Q 10/20 (2013.01)] | 22 Claims |
|
1. A system, comprising:
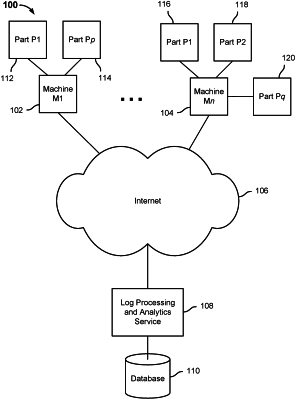
a communication interface configured to receive log data associated with a plurality of devices, the plurality of devices comprising a population of devices of a same device type each having a same target part subject to failure and the log data for each of at least a subset of the devices comprising ten thousand or more logged events per day; and
a processor coupled to the communication interface and configured to:
determine based on the received log data, for each of at least a subset of the plurality of devices, a replacement date on which the target part was replaced in that device;
parse the received log data to generate a structured dataset that includes for each device a plurality of sets of structured event data, each set of structured event data including for an event with which that set of structured event data is associated at least an event severity and an event description;
use the structured dataset to extract from the log data for said plurality of devices a set of structured logged event data with prescribed severity, each logged event in the set having a corresponding event type, the event type being determined at least in part by processing the event description data comprising the structured dataset to determine that a static portion of the data comprising the event description of the logged event is associated with the event type;
identify programmatically a subset of the logged event data as being associated with impending failure of the target part, at least in part by determining for each event type with respect to each device in said plurality of devices whether an event of that event type was logged for that device with a first prescribed daily frequency or number of occurrences within a first window before replacement of the target part in that device and determining that events of that event type were logged as having occurred with less than a second prescribed daily frequency or number of occurrences, the second prescribed daily frequency or number of occurrences being less than the first prescribed daily frequency or number of occurrences by a prescribed amount, within a second window following replacement of the target part in that device;
transform the subset of the logged event data into a normalized form at least in part by determining and storing a count of occurrences of logged events per day for each of a plurality of event types associated with predicting failure of the target part for each device on each of a plurality of days; and
use the transformed subset of the logged event data to train a machine learning model that can predict failure of the target part in a device based on the current event logs from that device, including by:
using the transformed subset of the logged event data to generate for each of a plurality of different machine learning algorithms a corresponding candidate machine learning model generated using that machine learning algorithm;
testing the resulting candidate machine learning models by using each to generate failure predictions based on a test dataset;
evaluating the respective candidate machine learning models using one or more performance metrics;
selecting the candidate machine learning model that exhibited the best performance as measured using the one or more performance metrics; and
deploying the selected candidate machine learning model to predict failure of the target part in a device based on the current event logs from that device.
|