| CPC G01N 33/66 (2013.01) [A61B 5/1495 (2013.01); B01L 3/523 (2013.01); G01N 21/78 (2013.01); G01N 27/3274 (2013.01); G01N 33/96 (2013.01); G16H 10/40 (2018.01); G16H 40/63 (2018.01); G16Z 99/00 (2019.02); A61B 5/14532 (2013.01); A61B 2560/0223 (2013.01); A61B 2562/08 (2013.01); B01L 2200/148 (2013.01); B01L 2300/022 (2013.01); B01L 2300/123 (2013.01); B01L 2400/0481 (2013.01); B01L 2400/0683 (2013.01); G01N 27/3273 (2013.01); G01N 33/48785 (2013.01); G01N 2201/12707 (2013.01); G01N 2496/80 (2013.01); Y10T 436/10 (2015.01); Y10T 436/107497 (2015.01)] | 20 Claims |
|
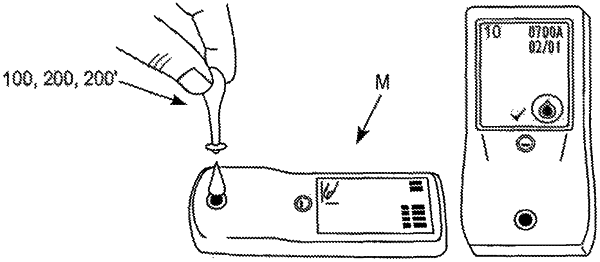
1. A method of verifying the accuracy of an analyte monitoring device through a control test, the method comprising:
receiving a fluid sample with the analyte monitoring device;
identifying the fluid sample as one of a first control solution and a second control solution with the analyte monitoring device automatically, wherein the first control solution is different from the second control solution;
analyzing the fluid sample to measure a concentration of an analyte contained therein;
comparing the measured concentration of the analyte in the fluid sample with control information; and
providing a signal to indicate whether the measured concentration falls within a range of acceptable analyte concentration values for the identified control solution.
|