| CPC C07D 487/04 (2013.01) [A61K 31/517 (2013.01); A61K 31/519 (2013.01); A61K 31/52 (2013.01); A61K 31/5377 (2013.01); A61K 31/7076 (2013.01); A61K 45/06 (2013.01); A61P 9/00 (2018.01); A61P 17/00 (2018.01); A61P 19/00 (2018.01); A61P 35/00 (2018.01); A61P 35/02 (2018.01); C07D 239/42 (2013.01); C07D 239/94 (2013.01); C07D 403/04 (2013.01); C07D 403/06 (2013.01); C07D 403/12 (2013.01); C07D 417/04 (2013.01); C07D 471/04 (2013.01); C07D 473/34 (2013.01); C07D 495/04 (2013.01); C07H 19/16 (2013.01); Y02A 50/30 (2018.01)] | 4 Claims |
|
1. A compound of formula VII, or pharmaceutically acceptable salt thereof,
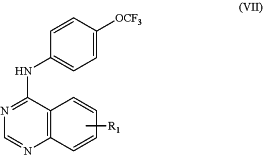 wherein
R1 is selected from a substituted C1-6alkyl, —C(O)Rx, (CH2)nC(O)Rx, (CH2)nC(O)ORx, (CH2)nC(O)NRxRx, or —(CH2)nC(O)NRxRxS(O)2Rx;
Rx, for each occurrence, is independently H, an optionally substituted C1-6alkyl, an optionally substituted C3-12cycloalkyl, an optionally substituted C3-12heterocycloalkyl, an optionally substituted C3-12aryl, an optionally substituted C3-12heteroaryl, NHNHR2, or C(NH)NH2;
R2 is H or C1-4 alkyl; and
each n is independently 0, 1 or 2,
wherein the substituted C1-6alkyl is not ethyl.
|