| CPC C07D 403/04 (2013.01) [C07D 213/69 (2013.01); C07D 213/70 (2013.01); C07D 213/74 (2013.01); C07D 491/052 (2013.01); C07D 498/08 (2013.01)] | 20 Claims |
|
1. A compound having a structure represented by a formula:
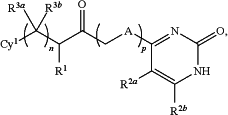 wherein n is 0, 1, or 2;
wherein p is 0 or 1;
wherein A is O, S, or NH;
wherein R1 is selected from hydrogen and C1-C4 alkyl;
wherein each of R2a and R2b is independently selected from hydrogen, halogen, C1-C6 alkyl, C1-C4 haloalkyl, C1-C8 alkoxy, C1-C8 haloalkoxy, C1-C8 cyanoalkoxy, —OCy2, —OAr1, —O(C1-C4 alkyl)OR10, —O(C1-C4 alkyl)Ar1, —CO2R10, and Cy2;
wherein each occurrence of R10, when present, is independently selected from hydrogen and C1-C4 alkyl;
wherein each occurrence of Ar1, when present, is independently selected from C2-C5 heteroaryl and C6-C12 aryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl;
wherein each occurrence of Cy2, when present, is independently selected from C3-C6 cycloalkyl, C3-C6 heterocycloalkyl, and phenyl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl;
or wherein R2a and R2b are covalently bonded and, together with the intermediate atoms, comprise a C5-C6 cycloalkyl, a C2-C5 heterocycloalkyl, a C6 aryl or a C2-C5 heteroaryl, and are substituted with 0, 1, 2, or 3 groups independently selected from selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl; and
wherein each of R3a and R3b, when present, is independently selected from hydrogen, halogen, C1-C4 alkyl, and C1-C4 haloalkyl;
or wherein R3a and R3b, when present, are covalently bonded and, together with the intermediate atoms, comprise a C3-C4 cycloalkyl substituted with 0, 1, 2, or 3 groups independently selected from selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, and C1-C4 aminoalkyl;
wherein Cy1 is selected from C2-C9 heteroaryl, C6 aryl, and adamantyl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —CN, —NH2, —OH, —NO2, C1-C4 alkyl, C2-C4 alkenyl, C1-C4 haloalkyl, C1-C4 cyanoalkyl, C1-C4 hydroxyalkyl, C1-C4 haloalkoxy, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, C1-C4 aminoalkyl, and C3-C6 cycloalkyl;
provided that when Cy1 is C2-C9 heteroaryl, then either (i) p is 1 and A is O or (ii) n is 1 or 2 and each of R3a and R3b are not hydrogen;
provided that when Cy1 is C6 aryl, then p is 1 and either (i) A is O or (ii) each of R2a and R2b is hydrogen and at least one of R3a and R3b is not hydrogen; and
provided that when Cy1 is
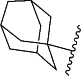 and p is 0, then n is 0 or 2,
or a pharmaceutically acceptable salt thereof.
|