| CPC A62D 1/0014 (2013.01) [A62D 1/0007 (2013.01)] | 4 Claims |
|
1. A fire extinguishing agent capable of extinguishing aluminum slag combustion, comprising the following raw materials: sulfate, chloride salt, a mineral, silica gel, a surfactant and stearate, wherein the fire extinguishing agent capable of extinguishing aluminum slag combustion is prepared by a preparation method comprising the following steps:
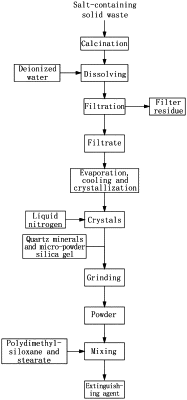
(1) calcinating solid waste containing sulfate and chloride salt, dissolving by adding water, performing filtration to obtain a filtrate, and performing evaporation and crystallization to obtain crystals:
(2) adding the crystals to liquid nitrogen for soaking and mixing the soaked crystals with the mineral and silica gel to prepare a powder, and
(3) mixing the powder, surfactant and stearate and drying an obtained mixture to obtain the fire extinguishing agent capable of extinguishing aluminum slag combustion;
wherein, the sulfate is sodium sulfate and calcium sulfate; the chloride salt is sodium chloride and calcium chloride; the surfactant is polydimethylsiloxane; the stearate is one of sodium stearate, magnesium stearate, calcium stearate and zinc stearate; the mineral is at least one of quartz sand, quartzite, silica and opal; the mass ratio of the powder, surfactant and stearate is 100:(1-5):(0.05-0.25); the mass ratio of the sodium sulfate, sodium chloride, calcium sulfate and calcium chloride is (50-80):(20-40):(2-10):(1-10); and wherein in step (2), the mass ratio of the crystals to the liquid nitrogen is 10:(1-3); and in step (2), the mass ratio of the crystals, the mineral and the silica gel is 100:(1-5):(1-2).
|