| CPC A61M 25/1029 (2013.01) [A61K 31/337 (2013.01); A61K 47/14 (2013.01); A61M 25/104 (2013.01); A61M 2025/1004 (2013.01); A61M 2025/1031 (2013.01); A61M 2025/105 (2013.01)] | 5 Claims |
|
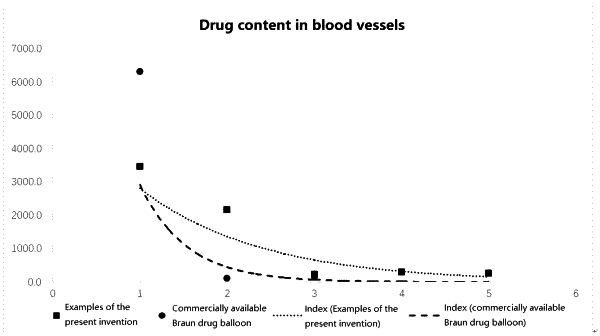
1. A drug-coated balloon comprising a surface liquefied drug coating and a balloon, wherein the drug coating consists of a lipophilic excipient and a drug, the drug accounts for 20% to 50% of the total weight of the drug coating, the lipophilic excipient accounts for 50% to 80% of the total weight of the drug coating, and the lipophilic excipient is a triglyceride.
|