| CPC A61K 47/6851 (2017.08) [A61K 31/519 (2013.01); A61K 31/553 (2013.01); A61K 47/6803 (2017.08); A61P 11/00 (2018.01); A61P 35/00 (2018.01); A61P 35/04 (2018.01)] | 15 Claims |
|
1. A method of treating a Trop-2 expressing cancer comprising:
administering to a human patient in need a therapeutically effective amount of an antibody-drug conjugate, and a therapeutically effective amount of an MCL-1 inhibitor;
wherein the antibody-drug conjugate comprises an anti-Trop-2 antibody and an anticancer agent that is SN-38; and
wherein the MCL-1 inhibitor is compound A:
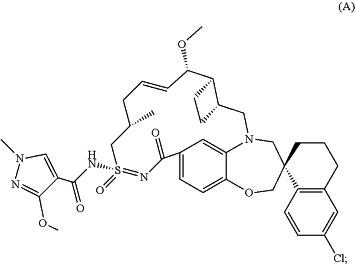 or a pharmaceutically acceptable salt thereof.
|