| CPC A61K 47/6815 (2017.08) [A61K 39/39558 (2013.01); A61K 47/6853 (2017.08); A61K 47/6857 (2017.08); C07K 16/2803 (2013.01); C07K 16/30 (2013.01); C07K 16/3007 (2013.01); C07K 16/3023 (2013.01); C12N 9/80 (2013.01); C07K 2317/22 (2013.01); C07K 2317/569 (2013.01); C07K 2317/90 (2013.01); C07K 2317/92 (2013.01)] | 26 Claims |
|
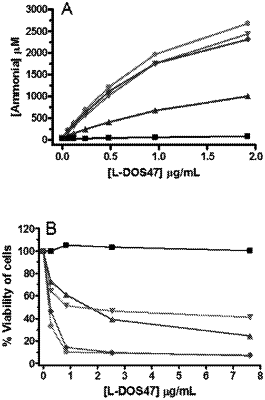
1. A pharmaceutical composition comprising:
a pharmaceutically acceptable aqueous solution suitable for intravenous injection, and
a single domain antibody-urease conjugate, wherein the conjugate has a conjugation ratio of 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 single domain antibody moieties per urease moiety, the single domain antibody having binding specificity to CEACAM6 and an amino acid sequence of SEQ ID NO: 1 or an amino acid sequence with at least 95% sequence identity with SEQ ID NO: 1, wherein the conjugate has urease activity;
wherein said composition is substantially free of unconjugated urease.
|