| CPC A61K 31/4192 (2013.01) [A61K 31/282 (2013.01); A61K 31/422 (2013.01); A61K 31/423 (2013.01); A61K 31/437 (2013.01); A61K 31/4439 (2013.01); A61K 31/4985 (2013.01); A61K 31/519 (2013.01); A61K 31/704 (2013.01); A61K 33/243 (2019.01); A61K 39/3955 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01)] | 11 Claims |
|
1. A compound represented by formula (Ia) or (Ib):
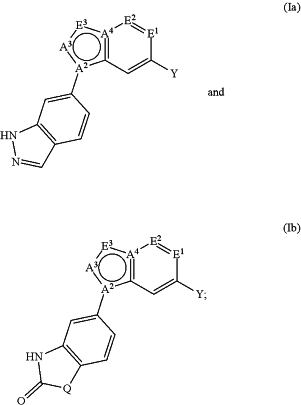 or a pharmaceutically acceptable salt, hydrate, N-oxide, or solvate thereof, wherein,
each of A2 and A4 is independently selected from the group consisting of C, CH and N;
A3 is selected from the group consisting of C(O), CH and N;
each E1 and E2 is independently selected from the group consisting of N, C(O) and CR1;
E3 is selected from the group consisting of N, NR1 and CR1;
at least two of A2, A3, A4, E1, E2 and E3 are N or NR1;
each R1 is independently selected from the group consisting of hydrogen, halogen, CN, NH2, NH—C1-C6 alkyl, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, and C1-C6 haloalkoxy, wherein NH2, NH—C1-C6 alkyl, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, and C1-C6 haloalkoxy are each substituted with from 0 to 3 R;
Y is an optionally substituted 5-6 membered heteroaryl or an optionally substituted 4-7 membered heterocycle, wherein said 5-6 membered heteroaryl and 4-7 membered heterocycle have from 1 to 3 ring heteroatoms independently selected from N, O, S, SO, and SO2;
Q is selected from the group consisting of NH, CH2, and O;
each R is independently selected from the group consisting of H, halogen, CN, NH2, NHRa, NRaRb, Rc, OH, ORa, SRa, S(O)2Ra, —Xa—NH2, —Xa—NHRa, —Xa—NRaRb, —Xa—OH, —Xa—ORa, —Xa—Ra, —Xa—SRa, and —Xa—S(O)2Ra;
each Ra, Rb and Rc is independently selected from the group consisting of C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 hydroxyalkyl, C3-7 cycloalkyl, C2-C6 alkenyl, and C2-C6 alkynyl, or Ra and Rb attached to N form a 4- to 7-membered ring; and
each Xa is independently selected from the group consisting of C(O), C1-C4 alkylene, —O—C1-C4 alkylene, and C1-C4 alkylene-O—.
|