| CPC H01M 8/188 (2013.01) [B01J 41/13 (2017.01); H01M 8/0245 (2013.01)] | 23 Claims |
|
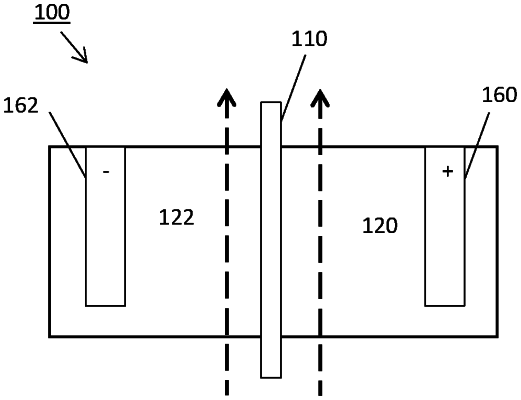
1. A flow battery comprising:
at least one rechargeable cell including an anolyte compartment configured to hold a first electrolyte having a first cation species;
a catholyte compartment configured to hold a second electrolyte having a second cation species; and
an anion exchange membrane positioned between the anolyte compartment and the catholyte compartment, configured to be ionically conductive between the first electrolyte and the second electrolyte, the anion exchange membrane formed from a microporous substrate having a thickness of less than 100 μm and a monomer having a cationic functional group and a cross-linking group polymerized to a surface of the microporous substrate, and the anion exchange membrane being fully cross-linked and having a steady state diffusivity of less than 0.4 ppm/hr/cm2 with respect to at least one of the first cation species and the second cation species.
|