| CPC H01M 10/0567 (2013.01) [H01G 11/60 (2013.01); H01G 11/62 (2013.01); H01G 11/64 (2013.01); H01M 4/505 (2013.01); H01M 4/525 (2013.01); H01M 10/0525 (2013.01); H01M 10/0569 (2013.01); H01G 9/20 (2013.01); H01M 2300/0025 (2013.01); Y02E 60/10 (2013.01); Y02E 60/13 (2013.01); Y02T 10/70 (2013.01)] | 8 Claims |
|
1. An electrolytic solution comprising:
a homocyclic compound other than aromatic compounds, the homocyclic compound containing at least one group selected from the group consisting of a nitrile group and an isocyanate group; and
a cyclic dicarbonyl compound; and
a solvent,
wherein the homocyclic compound is at least one selected from the group consisting of the following compounds
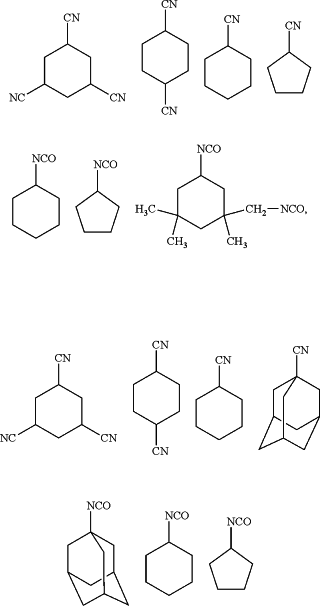 the amount of the homocyclic compound relative to the electrolytic solution is 0.001 to 5 mass %,
the cyclic dicarbonyl compound is at least one selected from the group consisting of lithium difluorooxalatoborate, trifluoromethylmaleic anhydride and maleic anhydride,
the amount of the cyclic dicarbonyl compound relative to the electrolytic solution is 0.001 to 5 mass %,
the solvent containing a fluorinated carbonate (2) containing at least one group selected from the group consisting of —CF3 and —CF2H, and a fluorinated saturated cyclic carbonate other than the fluorinated carbonate (2),
wherein a ratio by volume between the fluorinated carbonate (2) and the fluorinated saturated cyclic carbonate is (50 to 99)/(50 to 1), and
wherein the fluorinated carbonate (2) is at least one selected from the group consisting of:
a fluorinated carbonate represented by the following formula (2-1):
CF3—R21—OCOO—R22
(wherein R21 is a C1-C10 non-fluorinated or fluorinated alkylene group; and R22 is a C1-C10 non-fluorinated alkyl group);
a fluorinated carbonate represented by the following formula (2-2):
CF2H—R23—OCOO—R24
(wherein R23 is a C1-C10 non-fluorinated or fluorinated alkylene group; and R24 is a C1-C10 non-fluorinated alkyl group);
a fluorinated carbonate represented by the following formula (3-1):
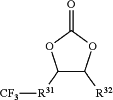 (wherein R31 is a single bond or a C1-C8 non-fluorinated or fluorinated alkylene group; and R32 is H or a C1-C3 non-fluorinated or fluorinated alkyl group); and
a fluorinated carbonate represented by the following formula (3-2):
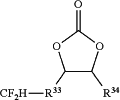 (wherein R33 is a single bond or a C1-C8 non-fluorinated or fluorinated alkylene group; and R34 is H or a C1-C3 non-fluorinated or fluorinated alkyl group).
|