| CPC C07D 403/04 (2013.01) [A61P 9/00 (2018.01); A61P 25/28 (2018.01); A61P 35/00 (2018.01); A61P 37/02 (2018.01); C07B 59/002 (2013.01); C07D 241/20 (2013.01); C07D 241/28 (2013.01); C07D 403/08 (2013.01); C07D 403/14 (2013.01); C07D 405/12 (2013.01); C07D 413/04 (2013.01); C07D 417/04 (2013.01); C07B 2200/05 (2013.01)] | 30 Claims |
|
1. A method of treating a disease or disorder in a patient, wherein the disease or disorder is associated with abnormal expression or activity of PI3Kγ kinase and is selected from the group consisting of colon cancer, gastric cancer, endometrial cancer, pancreatic cancer, renal cancer, breast cancer, skin cancer, head and neck squamous cell carcinoma, liver cancer, bladder cancer, astrocytoma, glioma, pancreatic cancer, and triple negative breast cancer, the method comprising administering to the patient a therapeutically effective amount of a compound of Formula (I):
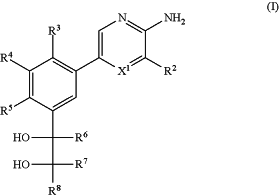 or a pharmaceutically acceptable salt thereof; wherein:
X1 is N or CR1;
R1 is selected from H, D, halo, C1-6 alkyl, C1-6 alkoxy, C2-6 alkenyl, C2-6 alkynyl, C1-6 haloalkyl, C1-6 haloalkoxy, CN, OH, and NH2;
R2 is C(O)NRc1Rd1;
R3, R4, and R5 are each independently selected from H, D, halo, CN, OH, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C1-6 alkoxy, C1-6 haloalkoxy, cyano-C1-6 alkyl, HO—C1-6 alkyl, C1-6 alkoxy-C1-6 alkyl, C3-6 cycloalkyl, amino, C1-6 alkylamino, di(C1-6 alkyl)amino, and C(O)NRcRd, wherein the C1-6 alkyl is optionally substituted by 1, 2, 3, 4, 5, or 6 D;
R6, R7, and R8 are each independently selected from H, D, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, (4-10 membered heterocycloalkyl)-C1-6 alkyl-, C(O)Rb3, C(O)NRc3Rd3, C(O)NRc3(ORa3), C(O)ORa3, C(═NRe3)Rb3, C(═NOH)Rb3, C(═NCN)Rb3, and C(═NRe3)NRc3Rd3, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of R6, R7, and R8 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RB substituents; and wherein the C1-6 haloalkyl of R6, R7 , or R8 is optionally substituted by 1, 2, 3, or 4 independently selected Y substituents;
each Y is independently selected from D, halo, C1-6 alkyl, and C1-6 haloalkyl;
or R6 and R7 substituents, together with the ring atoms to which they are attached, form a C3-10 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with 1, 2, 3, or 4 independently selected RB substituents;
or R7 and R8 substituents, together with the ring atoms to which they are attached, form a C3-10 cycloalkyl or 4-7 membered heterocycloalkyl, each of which is optionally substituted with 1, 2, 3, or 4 independently selected RB substituents;
Rc and Rd are each independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Rc and Rd, are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RM substituents;
each Rc1 and Rd1 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Rc1 and Rd1 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RA substituents;
or, any Rc1 and Rd1, attached to the same N atom, together with the N atom to which they are attached, form a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RA substituents;
each Ra3, Rb3, Rc3, and Rd3 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Ra3, Rb3, Rc3, and Rd3 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RB substituents;
or, any Rc3 and Rd3, attached to the same N atom, together with the N atom to which they are attached, form a 5- or 6-membered heteroaryl or a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 5- or 6-membered heteroaryl or 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RB substituents;
each Re3 is independently selected from H, OH, CN, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-10 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each RA is independently selected from D, halo, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, (4-10 membered heterocycloalkyl)-C1-6 alkyl-, CN, NO2, ORa4, SRa4, NHORa4, C(O)Rb4, C(O)NRc4Rd4, C(O)NRc4(ORb4), C(O)ORa4, OC(O)Rb4, OC(O)NRc4Rd4, NRc4Rd4, NRc4NRc4Rd4, NRc4C(O)Rb4, NRc4C(O)ORa4, NRc4C(O)NRc4Rd4, C(═NRe4)Rb4, C(═NOH)Rb4, C(═NRe4)NRc4Rd4, NRc4C(═NRe4)NRc4Rd4, NRc4C(═NRe4)Rb4, NRc4C(═NOH)NRc4Rd4, NRc4C(═NCN)NRc4Rd4, NRc4 S(O)Rb4, NRc4 S(O)NRc4Rd4, NRc4S(O)2Rb4, NRc4S(O)2NRc4Rd4, S(O)Rb4, S(O)NRc4Rd4, S(O)2Rb4, S(O)2NRc4Rd4, OS(O)(═NRe4)Rb4, OS(O)2Rb4, SF5, P(O)Rf4Rg4, OP(O)(ORh4)(ORi4), P(O)(ORh4)(ORi4), and BRj4Rk4, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of RA is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RD substituents;
each RB is independently selected from D, halo, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, (4-10 membered heterocycloalkyl)-C1-6 alkyl-, CN, NO2, ORa2, SRa2 NHORa2, C(O)Rb2, C(O)NRc2Rd2, C(O)NRc2(ORb2,) C(O)ORa2, OC(O)Rb2, OC(O)NRc2Rd2, NRc2Rd2, NRc2NRc2Rd2, NRc2C(O)Rb2, NRc2 C(O)ORa2, NRc2C(O)NRc2Rd2, C(═NRe2)Rb2, C(═NOH)Rb2, C(═NCN)Rb2, C(═NRe2)NRc2Rd2, NRc2C(═NRe2)NRc2Rd2, NRc2C(═NRe2)Rb2, NRc2C(═NOH)NRc2Rd2, NRc2C(═NCN)NRc2Rd2, NRc2S(O) Rb2, NRc2S(O)NRc2Rd2, NRc2S(O)2Rb2, NRc2S(O)2NRc2Rd2, S(O)Rb2, S(O)NRc2Rd2, S(O)2Rb2, S(O)2NRc2Rd2, OS(O)(═NRe2)Rb2, OS(O)2Rb2, SF5, P(O)Rf2Rg2, OP(O)(ORh2)(ORi2), P(O)(ORh2)(ORi2), and BRj2Rk2, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of RB is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RM substituents;
each Ra2, Rb2, and Rd2 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Ra2, Rb2, Rc2, and Rd2 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RM substituents;
or, any Rc2 and Rd2 attached to the same N atom, together with the N atom to which they are attached, form a 5- or 6-membered heteroaryl or a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 5- or 6-membered heteroaryl or 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RM substituents;
each Re2 is independently selected from H, OH, CN, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-; each Rf2 and Rg2 is independently selected from H, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rh2 and Ri2 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rj2 and Ri2 is independently selected from OH, C1-6 alkoxy, and C1-6 haloalkoxy;
or any Rj2 and Rk2 attached to the same B atom, together with the B atom to which they are attached, form a 5- or 6-membered heterocycloalkyl group optionally substituted with 1, 2, 3, or 4 substituents independently selected from C1-6 alkyl and C1-6 haloalkyl;
each Ra4, Rb4,Rc4, and Rd4 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Ra4, Rb4, Rc4, and Rd4 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RD substituents;
or, any Rc4 and Rd4 attached to the same N atom, together with the N atom to which they are attached, form a 5- or 6-membered heteroaryl or a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 5- or 6-membered heteroaryl or 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RD substituents;
each Re4 is independently selected from H, OH, CN, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-; each Rf4 and Rg4 is independently selected from H, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-; each Rh4 and Ri4 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Ri4 and Rk4 is independently selected from OH, C1-6 alkoxy, and C1-6 haloalkoxy; or any Rj4 and Rk4 attached to the same B atom, together with the B atom to which they are attached, form a 5- or 6-membered heterocycloalkyl group optionally substituted with 1, 2, 3, or 4 substituents independently selected from C1-6 alkyl and C1-6 haloalkyl;
each RD is independently selected from H, D, halo, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, (4-10 membered heterocycloalkyl)-C1-6 alkyl-, CN, NO2, ORa5, SRa5, NHORa5, C(O)Rb5, C(O)NRc5Rd5, C(O)NRc5(ORb5), C(O)ORa5, OC(O)Rb5, OC(O)NRc5Rd5, NRc5Rd5, NRc5NRc5Rd5, NRc5C(O)Rb5, NRc5C(O)ORa5, NRc5 C(O)NRc5Rd5, C(═NRe5)Rb5, C(═NOH)Rb5, C(═NCN)Rb5, C(═NRe5)NRc5Rd5, NRc5C(═NRe5)NRc5Rd5, NRc5C(═NRe5)Rb5, NRc5C(═NOH)NRc5Rd5, NRc5C(═NCN)NRc5Rd5, NRc5S(O)Rb5, NRc5 S(O)NRc5Rd5, NRc5S(O)2Rb5, NRc5S(O)2NRc5Rd5, S(O)Rb5, S(O)NRc5Rd5, S(O)2Rc5, S(O)2NRc5Rd5, OS(O)(═NRe5)Rb5, OS(O)2Rb5, SF5, P(O)Rf5Rg5, OP(O)(ORh5)(ORi5), P(O)(ORh5)(ORi5), and BRj5Rk5, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of RD are each optionally substituted with 1, 2, 3, or 4 independently selected RE substituents;
each Ra5, Rb5, Rc5, and Rd5 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Ra5, Rb5, Rc5, and Rd5 are each optionally substituted with 1, 2, 3, or 4 independently selected RE substituents;
or, any Rc5 and Rd5 attached to the same N atom, together with the N atom to which they are attached, form a 5- or 6-membered heteroaryl or a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 5- or 6-membered heteroaryl or 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, or 4 independently selected RE substituents; each Re5 is independently selected from H, OH, CN, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rf5 and Rg5 is independently selected from H, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rh5 and Ri5 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rj5 and Rk5 is independently selected from OH, C1-6 alkoxy, and C1-6 haloalkoxy; or any Ri5 and Rk5 attached to the same B atom, together with the B atom to which they are attached, form a 5- or 6-membered heterocycloalkyl group optionally substituted with 1, 2, 3, or 4 substituents independently selected from C1-6 alkyl and C1-6 haloalkyl;
each RE is independently selected from H, D, halo, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, CN, NO2, ORa6, SRa6, NHORa6, C(O)Rb6, C(O)NRc6Rd6, C(O)NRc6(ORb6), C(O)ORa6, OC(O)Rb6, OC(O)NRc6Rd6, NRc6Rd6, NRc6NRc6Rd6, NRc6C(O)Rb6, NRc6C(O)ORa6, NRc6C(O)NRc6Rd6C(═NRe6)Rb6, C(═NOH)Rb6, C(═NCN)Rb6, C(═NRe6)NRc6Rd6, NRc6C(═NRe6)NRc6Rd6, NRc6C(═NRe6)Rb6, NRc6C(═NOH)NRc6Rd6, NRc6C(═NCN)NRc6Rd6, NRc6S(O)Rb6, NRc6S(O)NRc6Rd6, NRc6S(O)2Rb6, NRc6S(O)2NRc6Rd6, S(O)Rb6, S(O)NRc6Rd6, S(O)2Rc6, S(O)2NRc6Rd6, OS(O)(═NRe6)Rb6, OS(O)2Rb6, SF5, P(O)Rf6Rg6, OP(O)(ORh6)(ORi6), P(O)(ORh6)(ORi6), and BRj6Rk6, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of RE are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RG substituents;
each Ra6, Rb6, Rc6 and Rd6 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-, wherein the C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl- of Ra6, Rb6, Rc6 and Rd6 are each optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RG substituents;
or, any Rc6 and Rd6 attached to the same N atom, together with the N atom to which they are attached, form a 5- or 6-membered heteroaryl or a 4-, 5-, 6-, or 7-membered heterocycloalkyl group, wherein the 5- or 6-membered heteroaryl or 4-, 5-, 6-, or 7-membered heterocycloalkyl group is optionally substituted with 1, 2, 3, 4, 5, 6, 7, or 8 independently selected RG substituents;
each Re6 is independently selected from H, OH, CN, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-; each Rf6 and Rg6 is independently selected from H, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rh6 and Ri6 is independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl-;
each Rh6 and Rk6 is independently selected from OH, C1-6 alkoxy, and C1-6 haloalkoxy; or any Rj6 and Rk6 attached to the same B atom, together with the B atom to which they are attached, form a 5- or 6-membered heterocycloalkyl group optionally substituted with 1, 2, 3, or 4 substituents independently selected from C1-6 alkyl and C1-6 haloalkyl; and
each RG is independently selected from H, D, halo, CN, NO2, SF5, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, and (4-10 membered heterocycloalkyl)-C1-6 alkyl; and each RM is independently selected from H, D, OH, NO2, CN, halo, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C1-6 haloalkyl, cyano-C1-6 alkyl, HO—C1-6 alkyl, C1-6 alkoxy-C1-6 alkyl, C6-10 aryl, C3-7 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl-, C3-7 cycloalkyl-C1-6 alkyl-, (5-10 membered heteroaryl)-C1-6 alkyl-, (4-10 membered heterocycloalkyl)-C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkoxy, amino, C1-6 alkylamino, di(C1-6 alkyl)amino, thio, C1-6 alkylthio, C1-6 alkylsulfinyl, C1-6 alkylsulfonyl, carbamyl, C1-6 alkylcarbamyl, di(C1-6 alkyl)carbamyl, carboxy, C1-6 alkylcarbonyl, C1-4 alkoxycarbonyl, C1-6 alkylcarbonylamino, C1-6 alkylsulfonylamino, aminosulfonyl, C1-6 alkylaminosulfonyl, di(C1-6 alkyl)aminosulfonyl, aminosulfonylamino, C1-6 alkyl aminosulfonylamino, di(C1-6 alkyl)aminosulfonylamino, aminocarbonylamino, C1-6 alkylaminocarbonylamino, and di(C1-6 alkyl)aminocarbonylamino.
|