| CPC A61N 1/3611 (2013.01) [A61N 1/0551 (2013.01); A61N 1/3601 (2013.01); A61N 1/36135 (2013.01); A61N 1/37288 (2013.01)] | 21 Claims |
|
1. A medical device for obstructive sleep apnea therapy, wherein the medical device is a first medical device implantable within a head or neck of a patient, and wherein the first medical device comprises:
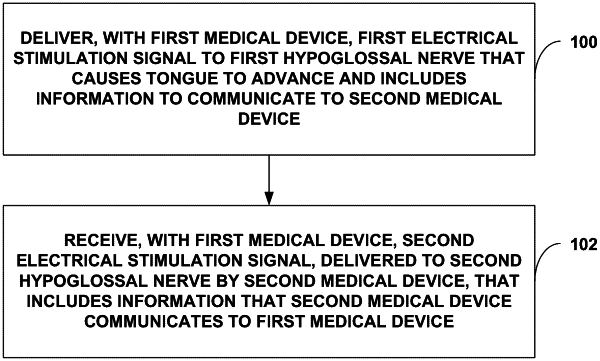
therapy delivery circuitry coupled to a first set of electrodes implantable proximate to a first hypoglossal nerve within a tongue of the patient and configured to deliver a first electrical stimulation signal to the first hypoglossal nerve that causes the tongue of the patient to advance and includes information to communicate to a second medical device implantable within the head or neck of the patient and coupled to a second set of electrodes implantable proximate to a second hypoglossal nerve within the tongue of the patient; and
sensing circuitry coupled to the first set of electrodes and configured to receive a second electrical stimulation signal, delivered to the second hypoglossal nerve by the second medical device, that includes information that the second medical device communicates to the first medical device.
|