| CPC A61K 31/5383 (2013.01) [A61K 9/1611 (2013.01); A61K 9/1623 (2013.01); A61K 9/1635 (2013.01); A61K 9/1652 (2013.01); A61K 47/02 (2013.01); A61K 47/12 (2013.01); A61K 47/14 (2013.01); A61K 47/22 (2013.01); A61K 47/26 (2013.01); A61K 47/38 (2013.01)] | 22 Claims |
|
1. A solid dosage form comprising a compound represented by formula (I):
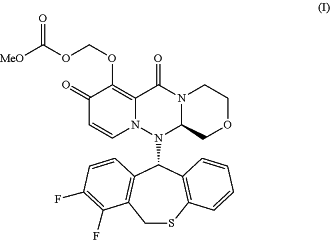 or a pharmaceutically acceptable salt thereof, and an alkali metal chloride,
wherein the solid dosage form is in the form of a granule.
|