| CPC A61K 31/495 (2013.01) [A61K 31/4409 (2013.01); A61K 45/06 (2013.01); A61P 3/06 (2018.01); C07C 237/20 (2013.01); C07D 213/64 (2013.01); A61K 2300/00 (2013.01); C07B 2200/07 (2013.01)] | 17 Claims |
|
1. A method of inhibiting PCSK9 in a subject with a metabolic disease, wherein the method comprises administering to the subject a compound of formula I:
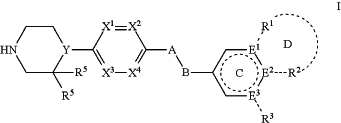 or a pharmaceutically acceptable salt, hydrate, or solvate thereof, wherein:
A is —SO2— and B is —NH—; or
A is —O— or —CH2— and B is —CH2— or absent;
ring C is a 5 or 6 membered heteroaryl or heterocyclic ring, or a 6 membered aryl ring;
E1, E2, and E3 are independently selected from C, CH, and N, wherein one of E1, E2, and E3 may be absent;
R1 and R2 are independently selected from the group consisting of H, lower alkyl, hydroxy, amino, aminoalkyl, hydroxyalkyl, haloalkyl, carboxy, —CONH2, —CON-alkyl, nitrile, —S-alkyl, —O-alkyl, acyl, and oxo; or
R1 and R2 with the atoms attached thereto form a 5-6 membered fused aryl, heteroaryl, carbocyclic or heterocyclic ring D containing 0-3 heteroatoms, where ring D may further be substituted at a position two atoms away from the juncture with ring C;
R3 is independently selected from the group consisting of H, lower alkyl, hydroxy, amino, aminoalkyl, hydroxyalkyl, haloalkyl, carboxy, —CONH2, —CON-alkyl, nitrile, —S-alkyl, —O-alkyl, acyl, and oxo;
R4 is selected from the group consisting of H, OH, halogen, and lower alkyl;
each R5 is hydrogen or taken together are oxo;
Y is selected from the group consisting of N and CH; and
X1, X2, X3, and X4 are independently selected from the group consisting of CH, C-R4, and N.
|