| CPC H01M 10/0562 (2013.01) [C01B 25/14 (2013.01); H01M 10/0525 (2013.01); C01P 2002/72 (2013.01); C01P 2002/74 (2013.01); C01P 2006/40 (2013.01); H01M 2300/0068 (2013.01)] | 12 Claims |
|
1. A method for producing a solid electrolyte material for a solid state battery, the solid electrolyte material having the following chemical formula XM2(PS4)3, where P is phosphorus, S is sulfur and X is lithium (Li), sodium (Na), silver (Ag) or magnesium (Mg0,5) and M is titanium (Ti), zirconium (Zr), germanium (Ge), silicon (Si), tin (Sn) or a mixture of X and aluminium (X+Al) and the method comprising:
mixing powders so as to obtain a powder mixture; and
sintering the powder mixture for a period of time equal to or greater than 75 hours and equal to or smaller than 500 hours at a sintering plateau temperature so as to obtain the solid electrolyte material;
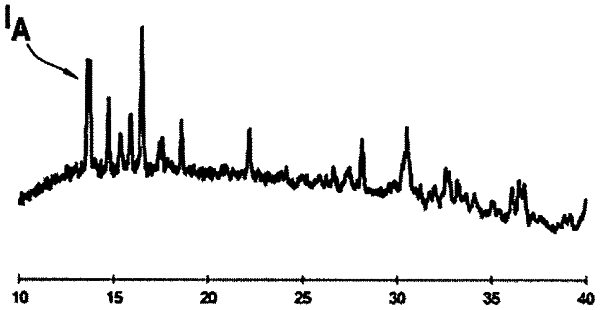
wherein the solid electrolyte material exhibits the peaks in positions of 2θ=) 13,64° (±1°,)16,48° (±1° and)22,18° (±1° in a X-ray diffraction measurement using CuKα line, where IA is the intensity in arbitrary units of the peak at)13,64° (±1° and IB is the intensity in arbitrary units of a peak at)23,34° (±1°, (IA−IB)/(IA+IB) >0(±1°,(IA−IB)/(IA+IB) >0.
|