| CPC C07D 487/14 (2013.01) [A61K 31/5025 (2013.01); A61K 31/53 (2013.01); A61K 31/5365 (2013.01); A61K 31/5383 (2013.01); A61K 31/551 (2013.01); A61P 31/16 (2018.01); C07D 471/14 (2013.01); C07D 498/14 (2013.01)] | 22 Claims |
|
1. A method of treating a viral infection, comprising administering to a subject a therapeutically effective amount of a compound of formula:
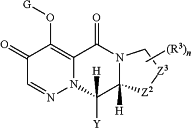 or a pharmaceutically acceptable salt thereof, wherein:
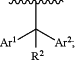
Y is a group of the formula
 G is H or a group selected from —C(O)R0, —C(O)—OR0, —C(RG)2—O—C(O)R0, —C(RG)2—O—C(O)—OR0, —P(═O)(OR0)2, —(CRG)2—O—P(═O)(OR0)2, —C(O)—N(R0)2, and —C(RG)2—O—C(O)N(R0)2,
where each R0 is independently H or a group selected from C1-C6 alkyl, phenyl, pyridyl, C3-C7 cycloalkyl, and a 3-6 membered heterocyclic ring containing one or two heteroatoms selected from N, O and S as ring members; and each R0 that is not H is optionally substituted with one or two groups selected from halo, CN, —OH, amino, C1-4 alkyl, COOR, phenyl, C1-4 alkoxy, C1-4 haloalkyl, and C1-4 haloalkoxy;
and each RG is independently selected from H and C1-4 alkyl;
Z2 is NR, O, S, or CH2;
Z3 is CH2, Q, —CH2—CH2—, -Q-CH2—, —CH2-Q-, —CH2-Q-CH2— or —CH2—CH2—CH2—;
Q is selected from —NR—, O, S, SO, and SO2;
R2 is selected from H, halo, CN, C1-4 alkyl optionally substituted with up to three groups independently selected from halo, CN, C1-4 alkyl, —OR, C1-4 haloalkoxy, —NR2, and C1-4 haloalkyl, OR, and C1-C4 haloalkyl;
each R3 is a substituent optionally present on any carbon atom of the ring containing Z2 and Z3, and is independently selected from halo, —OR, C1-4 haloalkyl, C1-4 haloalkoxy, oxo, CN, —NR2, and C1-4 alkyl optionally substituted with up to three groups independently selected from halo, CN, C1-4 alkyl, —OR, C1-4 haloalkoxy, —NR2, and C1-4 haloalkyl;
n is 0-2;
Ar1 and Ar2 each independently represent phenyl or a 5-6 membered heteroaryl ring containing 1-3 heteroatoms selected from N, O and S as ring members, and Ar1 and Ar2 are each independently substituted with up to three groups selected from halo, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C2-4 alkyne, and CN;
and Ar1 and Ar2 are optionally linked together by a bridge of the formula —C(RL)2-L- to form a tricyclic group, wherein Ar1 and Ar2 are each optionally substituted by up to two groups independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C2-4 alkyne, and CN;
R is independently at each occurrence H or C1-C4 alkyl optionally substituted with up to three groups independently selected from halo, OH, oxo, C1-4 alkyl, C1-4 alkoxy, C1-4 haloalkoxy, and C1-4 haloalkyl;
L is selected from S, S═O, SO2, O, NR, C(RL)2 and CF2; and
each RL is independently H or C1-2 alkyl.
|