| CPC C07D 257/10 (2013.01) [C07D 487/06 (2013.01)] | 25 Claims |
|
1. A process for preparing a tetra-substituted aminobiphenol macrocyclic ligand having a structure (I), comprising the step of treating a precursor compound having a structure (II) with a compound having a structure R6-L where L represents a leaving group (hereinafter compound (III)) in the presence of a base;
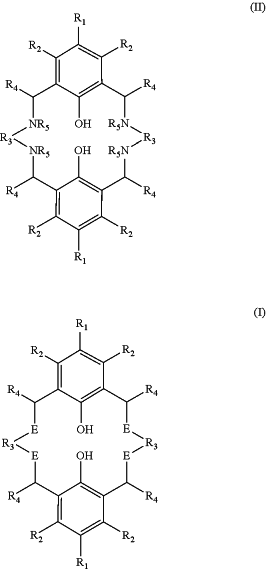 wherein R1 and R2 are independently selected from hydrogen, halide, a nitro group, a nitrile group, an imine group, —NCR13R14, an amine, an ether group —15 or —R16OR17, an ester group —OC(O)R10 or —C(O)OR10, an amido group —NR9C(O)R9 or —C(O)—NR9(R9), —COOH, —C(O)R15, —OP(O)(OR18)(OR19), —P(O)R20R21, a silyl group, a silyl ether group, a sulfoxide group, a sulfonyl group, a sulfinate group or an acetylide group or an optionally substituted alkyl, alkenyl, alkynyl, haloalkyl, aryl, heteroaryl, alkoxy, aryloxy, alkylthio, arylthio, alicyclic or heteroalicyclic group;
R3 is independently selected from 2,2-dimethylpropane-1,3-diyl, ethane-1,2-diyl, 2,2-fluoropropane-1,3-diyl, phenylene, cyclohexane-1,4-diyl, cyclohexane-1,2-diyl or biphenylene;
R4 is independently selected from hydrogen, or optionally substituted aliphatic, heteroaliphatic, alicyclic, heteroalicyclic, aryl, heteroaryl, alkylheteroaryl or alkylaryl;
R5 is independently selected from hydrogen, optionally substituted aliphatic, heteroaliphatic, alicyclic, alkanoate, arylate, carboxyl, heteroalicyclic, aryl, heteroaryl, alkylheteroaryl or alkylaryl,
or two R5 species may together be selected from optionally substituted alkylene, alkenylene or alkynylene, bonded to two different N groups of the compound of structure (II),
with the proviso that at least one of the species R5 is hydrogen;
and E is independently selected from NR5 and NR6, with the proviso that at least one of the species E is NR6;
wherein R6 is independently selected from optionally substituted aliphatic, alicyclic, heteroalicyclic, aryl, heteroaryl, ether, polyether, or optionally substituted alkylaryl or alkylheteroaryl;
wherein R9, R10, R13, R14, R18, R19, R20 and R21 are independently selected from hydrogen or an optionally substituted aliphatic, heteroaliphatic, alicyclic, heteroalicyclic, aryl or heteroaryl group and R15, R16 and R17 are independently selected from an optionally substituted aliphatic, heteroaliphatic, alicyclic, heteroalicyclic, aryl or heteroaryl group; and wherein the molar ratio of compound (III) to the number of NH sites in the compound of structure (II) is at least 0.6.
|