| CPC A61M 25/0133 (2013.01) [A61B 17/22 (2013.01); A61B 18/245 (2013.01); A61B 18/26 (2013.01); A61L 27/20 (2013.01); A61M 25/0009 (2013.01); A61M 25/0155 (2013.01); A61M 25/09 (2013.01); A61B 2017/22062 (2013.01); A61B 2018/0022 (2013.01); A61B 2018/00285 (2013.01); A61B 2018/2261 (2013.01); A61B 2018/2266 (2013.01); A61B 2018/2277 (2013.01); A61B 2018/2294 (2013.01); A61B 2018/263 (2013.01); A61B 2090/306 (2016.02); A61L 2400/12 (2013.01); A61M 2025/09008 (2013.01); A61M 2025/09183 (2013.01); A61M 2202/0415 (2013.01); A61M 2205/3592 (2013.01); A61M 2205/587 (2013.01)] | 20 Claims |
|
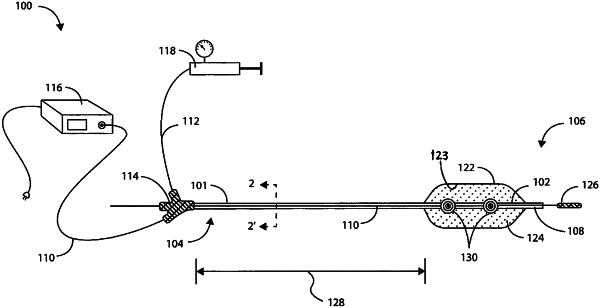
1. A catheter system for imparting pressure to induce fractures at a treatment site within or adjacent a blood vessel wall, comprising:
a catheter configured to advance to the treatment site, the catheter comprising an elongate shaft and a balloon that is coupled to the elongate shaft, the balloon being configured to expand to a first expanded configuration suitable for anchoring the catheter in position relative to the treatment site;
a fortified balloon inflation fluid that is configured to expand the balloon to the first expanded configuration, the fortified balloon inflation fluid including a base inflation fluid and a fortification component, the fortification component being configured to reduce a threshold for inducing plasma formation in the fortified balloon inflation fluid compared to the base inflation fluid, wherein the fortification component includes one of carbon and iron, and wherein the fortification component is provided as a fortification component coating that is disposed on a surface of a structure that is in fluid communication with the base inflation fluid; and
a first light guide that is disposed along the elongate shaft and at least partially within the balloon, the first light guide being configured to be in optical communication with a light source and the fortified balloon inflation fluid, the light source being configured to provide sub-millisecond pulses of a light to the first light guide so that plasma formation and rapid bubble formation occur in the fortified balloon inflation fluid, thereby imparting pressure waves upon the treatment site, the fortified balloon inflation fluid including absorptive agents having an absorption maxima of greater than 10 nanometers and less than 2.5 micrometers.
|