| CPC A61K 31/4439 (2013.01) [A61K 31/422 (2013.01); A61K 31/4245 (2013.01); A61K 31/433 (2013.01); A61K 45/06 (2013.01)] | 13 Claims |
|
1. A compound of formula (I), or a pharmaceutically acceptable salt thereof:
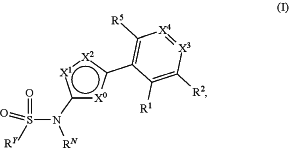 wherein either:
(i) X0=CRC, X1=N, —X2=O; or
(ii) X0=CRC, X1=O, X2=N; or
(iii) X0=S, X1=N, X2=N; or
(iv) X0=N, X1=N, X2=O; or
(v) X0=O, X1=N, X2=N;
where RC is H, CO2CH3 or Cl;
RN is H or methyl;
X3 is CR3 or N;
X4 is CR4 or N;
R1 to R5 are independently selected from:
(i) H;
(ii) halo;
(iii) cyano;
(iv) C1-3 alkyl, optionally substituted by one or more fluoro groups;
(v) (CH2)n0—C3-6 cycloalkyl, where n0=0 or 1;
(vi) (CH2)n1—C1-3 alkoxy, where n1=0 or 1, optionally substituted by one or more fluoro groups;
(vii) C1-3 alkylester;
(viii) (CH2)n2-phenyl, where n2=0-2; and
(ix) (CH2)n3—C5 heteroaryl, where n3=0-1, optionally substituted by methyl; and
RY is selected from:
(i) (CH2)n4-phenyl, where n4=0-2, where phenyl is optionally substituted by:
(a) C1-4 alkyl, optionally substituted by one or more fluoro groups;
(b) C1-4 alkoxy, optionally substituted by phenyl, or one or more fluoro groups;
(c) halo;
(d) cyano, nitro or amido;
(e) phenyl; or
(f) —(CH2)n5—, where n5 is 3 or 4;
(ii) pyridyl;
(iii) C3-4 alkyl;
(iv) (CH2)n6—C3-6 cycloalkyl, where n6=0-2;
(v) C6 heterocyclyl, optionally substituted by C1-4 alkylester; and
(vi) NHRYN, where RYN is selected from phenyl or cyclohexyl.
|