| CPC H10K 85/6572 (2023.02) [C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 487/04 (2013.01); C07D 491/048 (2013.01); C09K 11/06 (2013.01); H10K 85/615 (2023.02); H10K 85/657 (2023.02); H10K 85/6574 (2023.02); H10K 85/6576 (2023.02); C09K 2211/1018 (2013.01); H10K 50/11 (2023.02); H10K 2101/10 (2023.02); H10K 2101/30 (2023.02); H10K 2101/40 (2023.02)] | 17 Claims |
|
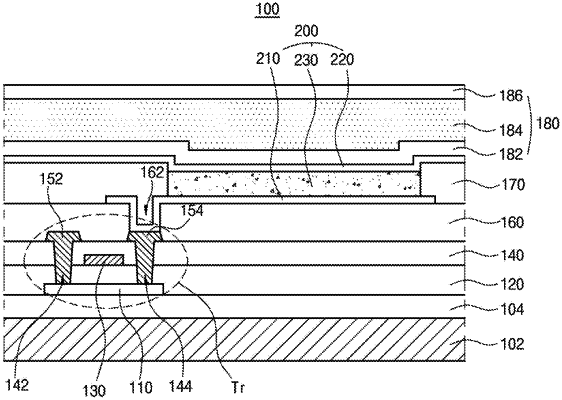
1. An organic light emitting diode, comprising:
first and second electrodes facing each other; and
at least one emitting unit disposed between the first and second electrodes and comprising an emitting material layer,
wherein the emitting material layer comprises a first compound, a second compound and a third compound,
wherein a Lowest Unoccupied Molecular Orbital (LUMO) energy level (LUMOH) of the first compound and a LUMO energy level (LUMOTD) of the second compound satisfies the following relationship in Equation (1),
wherein a Highest Occupied Molecular Orbital (HOMO) energy level (HOMOH) of the first compound, a HOMO energy level (HOMOTD) of the second compound and a HOMO energy level (HOMOFD) of the third compound satisfies the following relationship in Equation (2),
wherein an excited state singlet energy level (S1H) of the first compound is higher than an excited state singlet energy level (S1TD) of the second compound, and
wherein an excited state singlet energy level (S1TD) of the second compound is higher than an excited state singlet energy level (Sim) of the third compound:
|LUMOH|−|LUMOTD|≤0.8 eV (1)
|HOMOH|≥|HOMOTD|≥|HOMOFD| (2),
wherein the first compound is selected from a compound having the following structure of Chemical Formula 3:
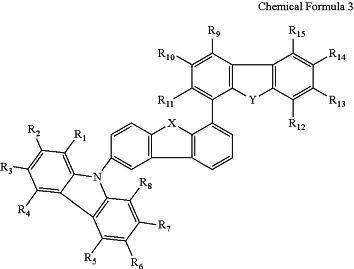 wherein each of R1 to R15 is independently hydrogen, deuterium, tritium, silyl group, C1˜C10 alkyl group, C1˜C10 alkoxy group, C1˜C10 alkyl amino group, C5˜C30 aryl group, C5˜C30 alkyl aryl group, C5˜C30 aryloxyl group, or C5˜C30 aryl amino group or two adjacent groups among R1 to R15 forms a fused aryl ring or a fused hetero aryl ring each of which is unsubstituted or substituted with C5˜C30 aryl group or C4˜C30 hetero aryl group; and
each of X and Y is independently oxygen (O) or sulfur (S);
wherein the second compound comprises:
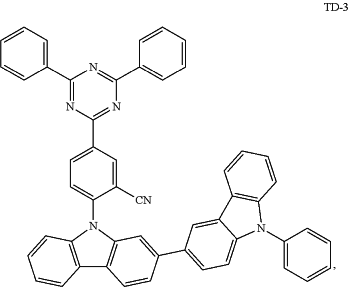 wherein the third compound has a quinoline-acridine core, and
wherein the third compound is selected from the group consisting of 5,12-dimethylquinolino(2,3-b)acridine-7,14(5H, 12H)-dione, 5,12-diethylquinolino(2,3-b)acridine-7,14(5H, 12H)-dione, 5,12-dibutyl-3,10-difluoroquinolino(2,3-b)acridine-7,14(5H, 12H)-dione, 5,12-dibutyl-3,10-bis(trifluromethyl)quinolino(2,3-b)acridine-7,14(5H, 12H)-dione and combinations thereof.
|