| CPC H10K 85/654 (2023.02) [C09D 11/03 (2013.01); C09D 11/102 (2013.01); C09D 11/38 (2013.01); C09D 11/52 (2013.01); H10K 71/15 (2023.02); H10K 85/113 (2023.02); H10K 85/151 (2023.02); H10K 85/215 (2023.02); H10K 85/60 (2023.02); H10K 85/621 (2023.02); H10K 85/624 (2023.02); H10K 85/626 (2023.02); H10K 85/655 (2023.02); H10K 85/6572 (2023.02); H10K 85/6576 (2023.02); H10K 30/00 (2023.02); H10K 2102/351 (2023.02)] | 20 Claims |
|
1. An organic photoactive layer composite ink, comprising
an electron donor material,
an electron acceptor material,
an organic solvent, and
an organic amine compound,
wherein the mass ratio of the electron donor material to the electron acceptor material is 5:1 to 1:5,
wherein the concentration of the electron donor material or the electron acceptor material is 5 to 20 mg/mL,
wherein the organic amine compound comprises an organic amine compound having a structure as shown in any one of Formulas (1), (2), (1-1), (2-1), and (4)-(8):
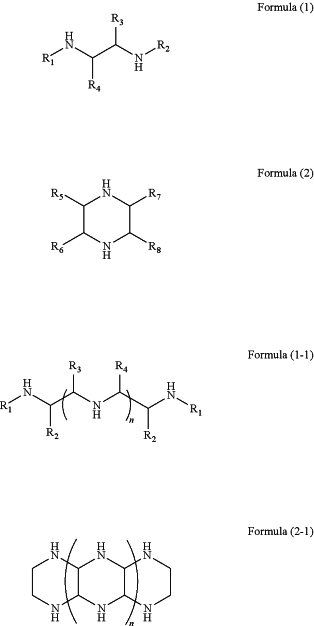 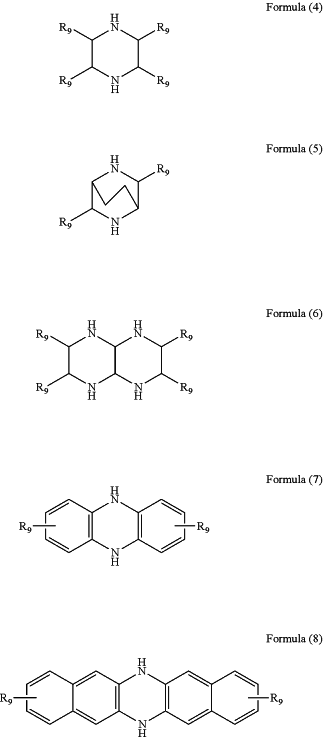 wherein, R1, R2, R3, R4, and R9 comprise hydrogen, substituted or unsubstituted C1 to C20 alkyl, C1 to C20 heteroalkyl, or substituted or unsubstituted modified aromatic or heteroaromatic π conjugated unit derivatives; R5, R6, R7 and R8 comprise hydrogen, substituted or unsubstituted C1 to C20 alkyl, C1 to C20 heteroalkyl, substituted or unsubstituted modified aromatic or heteroaromatic π conjugated unit derivatives, or a five or six membered cyclic structure formed by connecting any two substitution units in R5, R6, R7 and R8,
wherein the electron donor material comprises any one or a combination of two or more of poly(3-hexylthiophene), PTB7, PTB7-Th, PffBT4T-2OD, COOP-4HT-BDT, and structure variants thereof,
wherein the electron acceptor material comprises any one or a combination of two or more of a fullerene electron acceptor material, a fullerene derivative electron acceptor material and a non-fullerene electron acceptor material, and
wherein the mass of the organic amine compound is 0.01 wt % to 0.2 wt % of the total mass of the electron acceptor material and the electron donor material that comprises any one or a combination of two or more of PTB7, PTB7-Th, COOP-4HT-BDT, and structure variants thereof, or the mass of the organic amine compound is 1 wt % to 5 wt % of the total mass of the electron acceptor material and the electron donor material that comprises any one or a combination of two or more of poly(3-hexylthiophene), PffBT4T-2OD, and structure variants thereof.
|