| CPC G16H 50/20 (2018.01) [A61B 5/14503 (2013.01); A61B 5/14532 (2013.01); G06N 20/00 (2019.01); G16H 10/20 (2018.01); G16H 10/60 (2018.01); G16H 20/00 (2018.01); G16H 20/10 (2018.01); G16H 20/60 (2018.01); G16H 40/67 (2018.01)] | 20 Claims |
|
1. A computer-implemented method for managing blood glucose levels of a user, the method comprising:
causing a continuous glucose monitoring device comprising a subcutaneous sensor, in the user, to provide blood glucose values of the user;
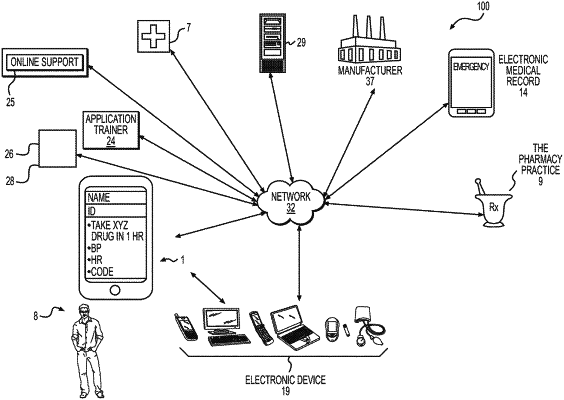
electronically receiving, at a server, using one or more processors, data including types and dosages of medications consumed by the user, and the blood glucose values of the user;
storing, using the one or more processors, in a database connected to the one or more processors, the data received at the server;
extracting health data, using the one or more processors, from the stored data, the health data comprising cohort data comprising blood glucose values of one or more other users;
inputting, using the one or more processors, the health data into one or more machine learning algorithms to generate a treatment plan for improving the blood glucose values of the user at an end of a treatment period, as compared to blood glucose values of the user at a beginning of the treatment period, the treatment plan for the user to achieve a goal based on the types and dosages of medications consumed by the user, wherein the treatment plan includes instructions for tasks to be performed by the user during a first subset of the treatment period, wherein the tasks include one or more of prescribed blood glucose measurement pairs to be measured before and after meals, prescribed timing and dosage of medication to be consumed by the user, a prescribed amount of carbohydrates to be consumed by the user, or prescribed exercise for the user to perform, wherein the one or more machine learning algorithms include one or more of artificial neural networks, Bayesian statistics, case-based reason, decision trees, inductive logic processing, Gaussian process regression, Gene expression programming, Logistic model trees, stochastic modeling, or statistical modeling;
verifying access rights of an electronic device of the user, wherein the verifying comprises compliance with at least one of a Health Insurance Portability and Accountability Act (HIPAA) privacy regulation, a legal regulation, a healthcare regulation, a user account, as an intended device, or a financial regulation;
outputting the treatment plan, using the one or more processors, to the user via the electronic device of the user;
electronically receiving, using the one or more processors, data relating to the treatment plan during the first subset of the treatment period, the data relating to the treatment plan including medications consumed by the user, amount of exercise performed by the user, and blood glucose levels of the user;
revising the treatment plan, using the one or more processors, for a subsequent subset of the treatment period based on at least one of a medication compliance, an exercise compliance, and identified patterns;
outputting the revised treatment plan to the user, using the one or more processors, via the electronic device, wherein the revised treatment plan includes one or more tasks to be performed by the user during the subsequent subset of the treatment period, wherein the tasks include a change in the one or more of the prescribed blood glucose measurement pairs to be measured before and after meals, the prescribed timing and dosage of medication to be consumed by the user, the prescribed amount of carbohydrates for the user to consume, or the prescribed exercise for the user to perform;
determining, using the one or more processors, based on the identified patterns, a trigger event that occurs before an adverse effect; and
sending a notification to the user, using the one or more processors, via the electronic device of the user upon detecting an instance of the trigger event, wherein the notification includes an identification of the trigger event to the user and an identification of the adverse effect.
|