|
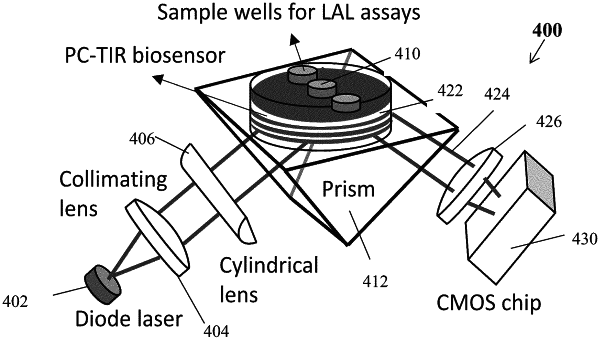
1. A Limulus Amoebocyte Lysate (LAL) endotoxin assay system comprising an open cavity photonic crystal total internal reflection (PC-TIR) biosensor, the PC-TIR biosensor further comprising a prism, a substrate, at least one dielectric layer on the substrate, at least one analyte well on the at least one dielectric layer, the substrate being coupled to the prism, the endotoxin assay system configured to detect endotoxin in an analyte in the at least one analyte well by monitoring changes in a refractive index of the analyte.
|