| CPC G01N 17/02 (2013.01) [C09D 5/086 (2013.01)] | 1 Claim |
|
1. An electrochemical method for a field monitoring of protective properties of an organic coating in a seawater environment, wherein
a peeling area of the organic coating being a parameter affecting an anticorrosive performance of the organic coating; and a cathodic disbonding failure of the organic coating being due to an effect of a cathodic reaction or cathodic reaction products on a bonding between the organic coating and a substrate metal, resulting in a separation of the organic coating from the substrate metal;
a corrosion potential of an anode and cathode electrode reaction being far from an equilibrium potential of the anode and cathode electrode reaction between two electrodes, so an inverse process of the anode and cathode electrode reaction between the two electrodes being ignored; and a kinetic formula of each electrode reaction being expressed by a Tafel formula
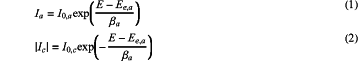 wherein Ia is an anode electrode reaction current density, I0,a is a current exchange density at an anode electrode reaction equilibrium, E is an electrode potential applied currently; Ee,a is an anode electrode reaction equilibrium potential, Ic is a cathode electrode reaction current density, I0,c is a cathode electrode reaction current exchange density at a cathode electrode reaction equilibrium, and Ee,c is a cathode electrode reaction equilibrium potential;
a velocity of a corrosion process of a corroded metal electrode being not controlled by a diffusion process of a cathode reaction; and a potential-current curve of the corroded metal electrode conforming to a three-parameter polarization curve equation:
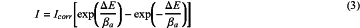 wherein, I is an external measured current density, ΔE is a polarization value of the corroded metal electrode, βa is a first anode tafel slope of the corroded metal electrode, βc is a first cathode tafel slope of the corroded metal electrode;
in a test electrolyte, a depolarizer being involved in the anode and cathode electrode reaction, and only an anodic reaction and only the cathodic reaction being carried out at the same time on each isolated electrode, instead of multiple electrode reactions occurring at the same time; and in an electrode reaction process, a mass transfer process being fast, and a concentration polarization being neglected; and
before the organic coating being peeled off, an anti-corrosion performance of the organic coating separating a metal matrix from a corrosive medium; wherein, the metal matrix produces no electrochemical corrosion, and the metal matrix under the organic coating is regarded as not participating in an electrochemical reaction; the electrochemical method comprises:
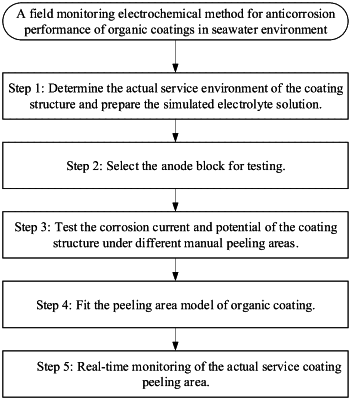
step 1: determining an actual service environment of a structure of the organic coating and preparing a simulated electrolyte solution; wherein
the actual service environment of the organic coating is sampled to measure types and concentrations of ions related to a corrosion reaction in the seawater environment; a concentration and a pH of a dissolved oxygen in seawater samples are measured; according to the actual service environment of the structure of the organic coating, an electrolyte solution of a test system is the simulated electrolyte solution or a real solution of the actual service environment of the structure of the organic coating; in the electrolyte solution, the types and the concentrations of the ions are consistent with the actual service environment to ensure the corrosion reaction and corrosion products of the structure of the organic coating are the same as the actual service environment, and the depolarizer controls the corrosion reaction with the metal matrix in the electrolyte solution;
step 2: selecting an anode block for testing; wherein
a coated metal sample is used as a working electrode; an inert metal electrode is used as an auxiliary electrode; a saturated silver chloride electrode is used as a reference electrode; and
the simulated electrolyte solution in the step 1 is used as a test electrolyte solution to constitute an electrochemical test three-electrode system;
a metal matrix sample is immersed in the test electrolyte solution to polarize for a first period of time, and an open circuit potential of the metal matrix sample is monitored; after the open circuit potential of the metal matrix sample is stabilized, a polarization curve of the metal matrix sample in the test electrolyte solution is measured; the open circuit potential of the metal matrix sample is converted into a standard hydrogen electrode potential according to a type of the reference electrode, and an electrode reaction standard potential table is searched to find a first metal with an electrode reaction potential much lower than the electrode reaction potential of the inert metal electrode to be tested (above 400 mV), wherein the inert metal electrode is used as a test anode metal; and
the test anode metal is made into a sample, and a working area of the test anode metal is designed to be about 2%-3% of a whole of a coated metal structure; the anode block is immersed in the test electrolyte solution for a polarization for a second period of time, and an open circuit potential of the anode block is measured; after the open circuit potential of the anode block is stable, a polarization curve of the anode block in the test electrolyte solution is measured;
step 3: testing a corrosion current and a potential of the structure of the organic coating under different manual peeling areas; wherein
the coated metal structure to be tested and the anode block are immersed into the test electrolyte solution and connected through a wire; under an action of a cathodic polarization, the anodic reaction of the coated metal structure is inhibited, and the cathodic reaction is promoted; in a whole galvanic corrosion system, the coated metal structure becomes a cathode, and the anode block becomes an anode; a non-resistance ammeter is connected in series to an anode and cathode circuit to measure a working current of the anode block, and an anode current is recorded when the anode current is stable; then, the reference electrode is immersed in the test electrolyte solution and connected in series with the coated metal structure to be tested; a potential difference between the reference electrode and the coated metal structure is measured by a voltmeter; a potential of the coated metal structure is calculated by adding a standard potential and the potential difference of the reference electrode; after a measurement is completed, a certain area of the organic coating is damaged artificially, and a second metal under the organic coating is immersed in the test electrolyte solution to make a direct contact with the test electrolyte solution; the measurement is repeated; and the certain area damaged artificially is gradually increased, and an anode working current and the potential of the coated metal structure are measured several times;
step 4: fitting a peeling area model of the organic coating; wherein
when the anode block and the coated metal structure to be tested exist as isolated electrodes in the test electrolyte solution, the corrosion potential is Ecorr1 and Ecorr2, corrosion current densities are Icorr1 and Icorr2, respectively; when the coated metal structure is connected with the anode block by the wire in the test electrolyte solution, the coated metal structure and the anode block form a corrosion galvanic couple; in the corrosion galvanic couple, the coated metal structure to be tested becomes the cathode, and the anode block becomes the anode; under a condition of ignoring the concentration polarization and a solution resistance, the coated metal structure is considered to be polarized to a same potential Eg after connecting with the anode block, wherein a polarization current density I1 of the anode block and a polarization current density I2 of the coated metal structure containing the organic coating are
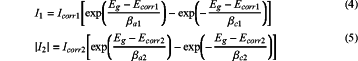 where, βa1 is a second anode Tafel slope on the anode block; βcl is a second cathode Tafel slope on the anode block; βa2 is a third anode Tafel slope on the coated metal structure; βc2 is a third cathode Tafel slope on the coated metal structure;
if areas of a contact between the test electrolyte solution of the anode block and the coated metal structure are A1 and A2, then a current ig in an external circuit of the whole galvanic corrosion system is
ig=I1A1=|I2|A2 (6)
since the corrosion potential of the anode block is much lower than the corrosion potential of the coated metal structure, Eg is far away from Ecorr2 and close to Ecorr1; therefore, the anodic reaction on a surface of the coated metal structure is ignored, but the cathodic reaction on a surface of the anode block is not ignored; thus, equation (6) is simplified as
ig=Ia1A1−|Icl|A1=|Ia2|A2 (7)
where, Ia1 is an anode dissolution current density after the contact between the anode block and the coated metal structure, |Ic1| and |Ia2| are an absolute value of a cathodic reduction current density of the depolarizer on the anode block and an absolute value of a cathodic reduction current density of the depolarizer on the coated metal structure after the contact between the anode block and the coated metal structure, respectively;
substituting equations (1) and (2) into equation (7), the following equation can be solved:
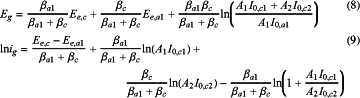 where, Ee,c is a cathodic reaction equilibrium potential of the depolarizer, Ee,a1 is an anode reaction equilibrium potential on the anode block, I0,a1 is an anode reaction exchange current density on the anode block, I0,cl is a cathodic reaction exchange current density on the anode block, and I0,c2 is a cathodic reaction exchange current density on the coated metal structure;
equation (8) is simplified as
Eg=a+bln(c+A2) (10)
wherein, a, b, and c are constants;
A2 gradually increases with a peeling of the organic coating; when A2
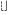 A1, equation (9) is simplified as follows: A1, equation (9) is simplified as follows:lnig=e+fln(A2) (11)
wherein, e and f are constants;
equations (10) and (11) describe a relationship between a potential and current and the peeling area of the organic coating; wherein, the equation (10) is logarithmic and a slope gradually decreases with an increase of the peeling area; the equation (10) is suitable for characterizing an early service period of the organic coating; the equation (11) is a power function; the equation (11) is only available when the peeling area is large; the equation (11) is suitable for characterizing a later service period of the organic coating; data of the potential and current measured in the step 3 are used to fit parameters of the equations (10) and (11), and a relationship model between the peeling area of the organic coating and the potential and current is obtained; and
step 5: real-time monitoring of an actual service coating peeling area; wherein due to a low resistivity of the seawater environment, the anode block is connected to the coated metal structure with a seawater medium and a small structure volume; the non-resistance ammeter, the reference electrode, and the voltmeter are connected in a loop to realize a function of estimating the peeling area of the organic coating by a real-time monitoring of a current or a potential; a specific method is to connect a zero-resistance galvanometer, the reference electrode, and the voltmeter in the anode and cathode circuit to realize the function of real-time monitoring the anode working current or potential; after an anode current and a mixing potential are obtained, the peeling area of the organic coating is backward deduced according to the equations (10) and (11) to realize the function of the real-time monitoring of a protection performance of the organic coating.
|