| CPC C12P 21/005 (2013.01) [A61K 31/70 (2013.01); A61K 31/7024 (2013.01); A61K 35/17 (2013.01); A61P 35/02 (2018.01); C12N 5/0636 (2013.01); C12N 2500/34 (2013.01)] | 8 Claims |
|
1. A method of producing T cells having reduced surface fucosylation, the method comprising:
culturing T cells in the presence of an effective amount of a fucose analog in a cell culture medium; wherein
said fucose analog is selected from the group consisting of formulae (I) and (II):
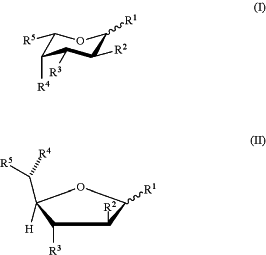 or a pharmaceutically acceptable salt or solvate form thereof, wherein each of formula (I) or (II) can be the alpha or beta anomer or the corresponding aldose form;
R2 is halogen; each of R1, R3, and R4 is independently —OH or a hydrolyzable ester group; and R5 is —CH3, or
each of R1, R2, R3, and R4 is independently —OH or a hydrolyzable ester group; and R5 is —C≡CH;
and
wherein said T cells cultured in the presence of the effective amount of said fucose analog have at least about 80% reduced surface fucosylation relative to T cells cultured in the absence of said fucose analog; and wherein the T cells having reduced surface fucosylation are configured to be used in an adoptive cell therapy.
|