| CPC C07K 7/08 (2013.01) [A61K 38/00 (2013.01)] | 12 Claims |
|
1. A method for treating Alzheimer's disease or type 2 diabetes, wherein said method comprises administering, to a patient in need of such treatment, a peptide having an amino acid sequence according to formula o
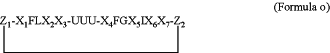 wherein
Z1 and Z2 are selected from the following pairs
a) cysteine and cysteine,
b) aspartic acid and lysine, or lysine and aspartic acid,
c) aspartic acid and ornithine, or ornithine and aspartic acid,
d) aspartic acid and 2,4-diaminobutyric acid, or 2,4-diaminobutyric acid and aspartic acid,
e) aspartic acid and 2,3-diaminopropionic acid, or 2,3-diaminopropionic acid and aspartic acid,
f) glutamic acid and lysine, or lysine and glutamic acid,
g) glutamic acid and ornithine, or ornithine and glutamic acid,
h) glutamic acid and 2,4-diaminobutyric acid, or 2,4-diaminobutyric acid and glutamic acid,
i) glutamic acid and 2,3-diaminopropionic acid, or 2,3-diaminopropionic acid and glutamic acid;
with
 denoting a covalent bond between Z1 and Z2, thus providing for a cyclization of the peptide; denoting a covalent bond between Z1 and Z2, thus providing for a cyclization of the peptide;X1, X2, X3, X4, X5, X6, and X7 are, independently at each occurrence, selected from glycine, asparagine, valine, histidine, leucine, serine, alanine, and threonine;
F is, independently at each occurrence, phenylalanine;
L is leucine;
U is, independently at each occurrence, selected from arginine, homoarginine, citrulline, ornithine, lysine, and norleucine;
G is glycine;
I is isoleucine;
wherein Z1, Z2, X1-X7, F, L, U, G and I are L-amino acid residues or D-amino acid residues, or some of Z1, Z2, X1-X7, F, L, U, G and I are L-amino acid residues and others are D-amino acid residues;
and pharmaceutically acceptable salts, esters, solvates, polymorphs and modified forms thereof.
|