| CPC C07D 487/04 (2013.01) [A61K 31/519 (2013.01); A61K 45/06 (2013.01); A61P 33/02 (2018.01); C07C 57/15 (2013.01); C07B 2200/13 (2013.01)] | 14 Claims |
|
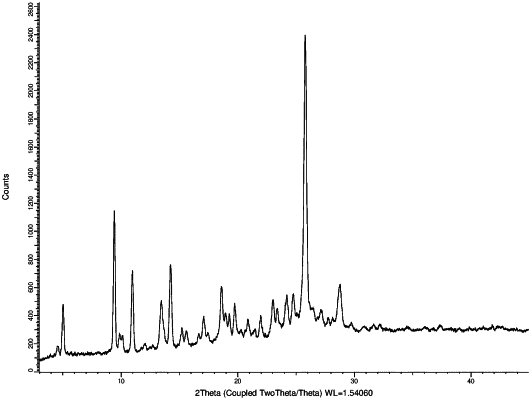
1. A co-crystal comprising N-(4-fluoro-3-(6-(3-methylpyridin-2-yl)[2,4]triazolo[1,5- a]pyrimidin-2-yl)phenyl)-2,4-dimethyloxazole-5-carboxamide and fumaric acid; wherein said co-crystal is characterized by:
(i) an X-ray powder diffraction pattern comprising three or more 2θ peaks selected from 24.7°±0.2°, 23.9°±0.2°, 16.0°±0.2° (2θ) and 13.4°±0.2° (2θ);
(ii) an X-ray powder diffraction pattern comprising three or more three or more 2θ peaks selected from 27.6°±0.2°, 14.8°±0.2°, 9.8°±0.2°, 11.3°±0.2°, 24.6°±0.2°, 25.0°±0.2° and 26.5°±0.2° (2θ); or
(iii) an X-ray powder diffraction pattern comprising three or more 2θ peaks selected from 13.5°±0.2°, 3.8°±0.2°, 17.2°±0.2°and 26.6°±0.2 (2θ);
when measured with a CuKα radiation at a wavelength of 0.15 nm at room temperature.
|