| CPC C07D 403/12 (2013.01) [C07B 2200/05 (2013.01)] | 12 Claims |
|
1. A compound of formula (I) or an optical isomer, a pharmaceutically acceptable salt, a hydrate or a solvate thereof:
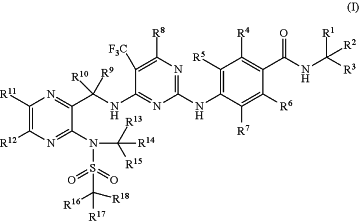 wherein, R1-R18 are each independently selected from hydrogen and deuterium, with the proviso that not all of them are hydrogen at the same time.
|