| CPC C07D 401/14 (2013.01) [A61P 27/06 (2018.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01)] | 27 Claims |
|
1. A method of treating an ocular disorder in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a compound of Formula (I):
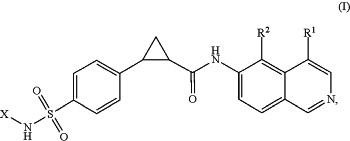 or a pharmaceutically acceptable salt thereof,
wherein
R1 is H, halo, —CN, —C1-6 alkyl, —C1-6 alkoxyl, —C1-6 haloalkyl, —C1-6 haloalkoxyl;
R2 is H or halo;
X is —(C1-5 heteroaryl)-R3; and
R3 is —C2-6 heterocyclyl, or —C1-6 heteroalkyl.
|