| CPC C07D 401/04 (2013.01) [A61K 31/4439 (2013.01); C07B 2200/13 (2013.01)] | 40 Claims |
|
1. A method of treating a hematologic malignancy in a mammal having said hematologic malignancy, comprising administering to the mammal a pharmaceutical composition comprising a therapeutically effective amount of a crystalline form of 1-(2R,4R)-2-(1H-benzo[d]imidazol-2-yl)-1-methylpiperidin-4-yl)-3-(4-cyanophenyl)urea maleate, having the structure:
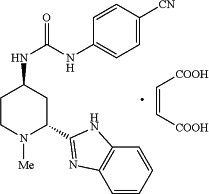 and having a powder X-ray diffraction pattern comprising peaks at 2θ values of: 11.6, 12.1 and 19.6°2θ±0.2°2θ, and a pharmaceutically acceptable excipient.
|