|
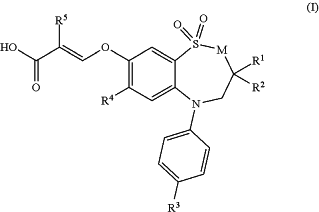
1. A method for treating a disease or disorder in a subject, the method comprising administering to the subject a therapeutically effective amount of a compound of formula (I),
wherein M, R1, R2, R3, R4 and R5 are as indicated in Table 1 below:
| TABLE 1 |
|
|
| M |
R1 |
R2 |
R3 |
R4 |
R5 |
|
|
| CH2 |
CH2CH2CH2CH3 |
CH2CH2CH2CH3 |
F |
SCH3 |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
H |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH3 |
F |
| CH2 |
CH2CH2CH3 |
CH2CH3 |
H |
SCH3 |
H |
| CH2 |
CH2CH2CH3 |
CH2CH3 |
H |
SCH3 |
F |
| CH2 |
CH(CH3)2 |
CH2CH3 |
H |
SCH3 |
H |
| CH2 |
CH(CH3)2 |
CH2CH3 |
H |
SCH3 |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
H |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
SCH2CH3 |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
SCH2CH3 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
SCH2CH3 |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
SCH2CH3 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
SCH2CH3 |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
H |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
Cl |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
Cl |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
Cl |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
Cl |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
Cl |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
H |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
N(CH3)2 |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
H |
N(CH3)2 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
H |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
N(CH3)2 |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
H |
N(CH3)2 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
H |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
F |
N(CH3)2 |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
SCH3 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
SCH3 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
SCH3 |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
Cl |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
Cl |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
Cl |
F |
| CH2 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
N(CH3)2 |
F |
| NH |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
N(CH3)2 |
F |
| NCH3 |
CH2CH2CH2CH3 |
CH2CH3 |
Cl |
N(CH3)2 |
F |
|
|
| |
|