| CPC A61M 31/002 (2013.01) [A61K 9/0034 (2013.01); A61K 9/2072 (2013.01); A61K 31/00 (2013.01); A61K 31/167 (2013.01); A61L 31/16 (2013.01); A61L 31/18 (2013.01); A61M 31/00 (2013.01); A61F 2/042 (2013.01); A61L 2300/402 (2013.01); A61L 2300/602 (2013.01); A61L 2430/22 (2013.01)] | 29 Claims |
|
1. An intravesical drug delivery device, comprising:
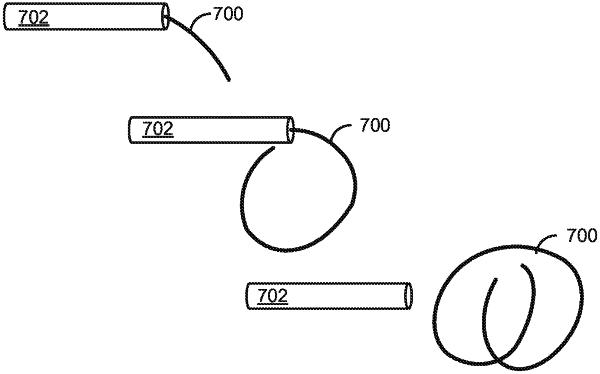
an elongated elastic body comprising a drug reservoir lumen defined therein; and
a drug formulation disposed within the drug reservoir lumen, the drug formulation comprising a drug which comprises a kinase inhibitor,
wherein the elongated elastic body has a coiled retention shape having (i) dimensions that provide intravesical mobility and prevent voiding of the device through the urethra, and (ii) a maximum dimension in any direction of 6 cm or less when in an uncompressed state, and
wherein the device exerts a maximum acting force less than 1 N when the device is compressed from the retention shape to a shape having a maximum dimension in any direction of 3 cm or less.
|