| CPC A61K 31/519 (2013.01) [A61P 35/04 (2018.01); C07K 16/2818 (2013.01); C07K 16/2827 (2013.01)] | 43 Claims |
|
1. A method of treating a KRas G12C-associated cancer in a subject in need thereof, comprising administering to the subject a therapeutically effective amount of a combination of a PD-1/PD-L1 inhibitor selected from the group consisting of nivolumab, pembrolizumab, cemiplimab, tislelizumab, atezolizumab, avelumab, and durvalumab, and a compound of formula:
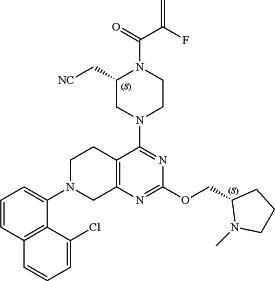 or a pharmaceutically acceptable salt thereof.
|
|
43. A kit comprising: a) a pharmaceutical composition comprising a PD-1/PD-L1 inhibitor selected from the group consisting of nivolumab, pembrolizumab, cemiplimab, tislelizumab, atezolizumab, avelumab, and durvalumab, and b) a pharmaceutical composition comprising a compound of formula:
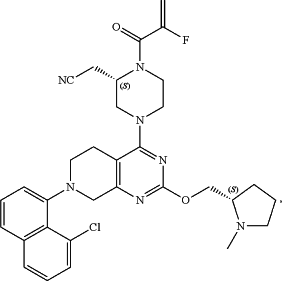 or a pharmaceutically acceptable salt thereof, for treating a KRas G12C cancer in a subject.
|