| CPC A61K 31/444 (2013.01) [A61K 31/282 (2013.01); A61K 31/337 (2013.01); A61K 31/4745 (2013.01); A61K 31/506 (2013.01); A61K 31/513 (2013.01); A61K 31/519 (2013.01); A61K 31/53 (2013.01); A61K 31/555 (2013.01); A61K 31/7068 (2013.01); A61K 38/19 (2013.01); A61K 39/395 (2013.01); A61K 45/06 (2013.01)] | 23 Claims |
|
1. A method for controlling an adenocarcinoma in a patient comprising administering to the patient in need thereof an effective amount of a pharmaceutical combination comprising a compound of Formula I:
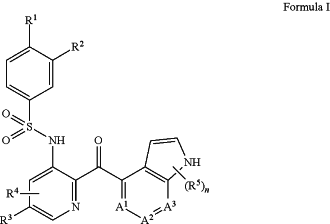 or a pharmaceutically acceptable salt thereof,
wherein
R1 is halogen or C1-6 alkyl;
R2 is hydrogen, halogen, C1-6 alkyl, C1-6 alkoxy, C1-6 haloalkyl, C1-6 haloalkoxy, or —CN;
R3 is hydrogen, halogen, or C1-6 alkyl;
R4 is hydrogen, halogen, or C1-6 alkyl;
each R5 is independently C1-6 alkyl, —OH, or —NH2;
n is 0, 1, 2, or 3; and
each of A1, A2, and A3 is —CH— or —N—, where at least one of A1, A2, or A3 is —N—; and
one or more additional therapeutic compound, wherein the pharmaceutical combination comprises a fixed dose combination or separate doses,
wherein the one or more additional therapeutic compound is selected from one or more of a Btk tyrosine kinase inhibitor, an Erbb2 tyrosine kinase receptor inhibitor; an Erbb4 tyrosine kinase receptor inhibitor, a thymidylate synthase inhibitor, an EGFR tyrosine kinase receptor inhibitor, an Epidermal growth factor antagonist, a Fyn tyrosine kinase inhibitor, a kit tyrosine kinase inhibitor, a Lyn tyrosine kinase inhibitor, a NK cell receptor modulator, a PDGF receptor antagonist, a PARP inhibitor, a poly ADP ribose polymerase inhibitor, a poly ADP ribose polymerase 1 inhibitor, a poly ADP ribose polymerase 2 inhibitor, a poly ADP ribose polymerase 3 inhibitor, a galactosyltransferase modulator, a dihydropyrimidine dehydrogenase inhibitor, an orotate phosphoribosyltransferase inhibitor, a telomerase modulator, a mucin 1 inhibitor, a mucin inhibitor, a secretin agonist, a TNF related apoptosis inducing ligand modulator, an IL17 gene stimulator, an interleukin 17E ligand, a Neurokinin receptor agonist, a cyclin G1 inhibitor, a checkpoint inhibitor, a PD-1 inhibitor, a PD-L1 inhibitor, a CTLA4 inhibitor, a topoisomerase I inhibitor, an Alk-5 protein kinase inhibitor, a connective tissue growth factor ligand inhibitor, a notch-2 receptor antagonist, a notch-3 receptor antagonist, a hyaluronidase stimulator, a MEK-1 protein kinase inhibitor; MEK-2 protein kinase inhibitor, a GM-CSF receptor modulator; TNF alpha ligand modulator, a mesothelin modulator, an asparaginase stimulator, a caspase-3 stimulator; caspase-9 stimulator, a PKN3 gene inhibitor, a hedgehog protein inhibitor; Smoothened receptor antagonist, an AKT1 gene inhibitor, a DHFR inhibitor, a thymidine kinase stimulator, a CD29 modulator, a fibronectin modulator, an interleukin-2 ligand, a serine protease inhibitor, a D40LG gene stimulator; TNFSF9 gene stimulator, a 2-oxoglutarate dehydrogenase inhibitor, a TGF-beta type II receptor antagonist, an Erbb3 tyrosine kinase receptor inhibitor, a cholecystokinin CCK2 receptor antagonist, a Wilms tumor protein modulator, a Ras GTPase modulator, an histone deacetylase inhibitor, a cyclin-dependent kinase 4 inhibitor A modulator, an estrogen receptor beta modulator, a 4-1BB inhibitor, a 4-1BBL inhibitor, a PD-L2 inhibitor, a B7-H3 inhibitor, a B7-H4 inhibitor, a BTLA inhibitor, a HVEM inhibitor, aTIM3 inhibitor, a GAL9 inhibitor, a LAG3 inhibitor, a VISTA inhibitor, a KIR inhibitor, a 2B4 inhibitor, a CD160 inhibitor and a CD66e modulator.
|