| CPC A61F 2/2418 (2013.01) [A61F 2/07 (2013.01); A61F 2/82 (2013.01); A61F 2/856 (2013.01); A61F 2/962 (2013.01); A61F 2/24 (2013.01); A61F 2/86 (2013.01); A61F 2/89 (2013.01); A61F 2/90 (2013.01); A61F 2002/061 (2013.01); A61F 2002/067 (2013.01); A61F 2210/00 (2013.01); A61F 2210/0014 (2013.01); A61F 2210/0057 (2013.01); A61F 2220/0008 (2013.01); A61F 2220/0016 (2013.01); A61F 2220/0025 (2013.01); A61F 2220/0058 (2013.01); A61F 2220/0075 (2013.01); A61F 2230/001 (2013.01); A61F 2230/0034 (2013.01); A61F 2230/0067 (2013.01); A61F 2250/0039 (2013.01); A61F 2250/0048 (2013.01); A61F 2250/0069 (2013.01)] | 27 Claims |
|
1. A heart valve assembly comprising:
a polymeric covering housing prosthetic heart valve leaflets, wherein the polymeric covering extends about the prosthetic heart valve leaflets for providing sealing to the prosthetic heart valve leaflets;
a metallic balloon-expandable stent defining an open cell configuration, and being secured to the polymeric covering, and being configured with a radially compressed orientation and a radially expanded orientation; and
a sealing material positioned externally to and in contact with the stent for providing sealing between the stent and a patient's anatomical wall to prevent paravalvular leaks,
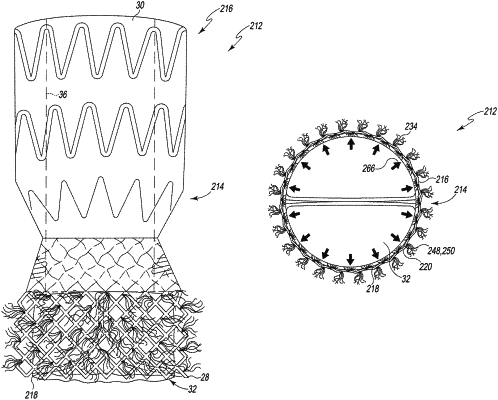
wherein the sealing material defines a porous, fibrous seal that is porous through a thickness of the sealing material such that portions of the stent behind the sealing material are uncovered before the heart valve assembly is deployed,
wherein the fibrous seal includes outwardly extending arcuate fibers,
wherein the heart valve assembly is sized and shaped for endovascular delivery through a femoral artery of the patient,
wherein expansion of the stent by balloon expansion from the radially compressed orientation to the radially expanded orientation is configured to press the outwardly extending fibers into engagement with native leaflets of the aorta of the patient such that the porosity is reduced, creating a paravalvular seal about the stent with the polymeric covering due to the reduction in porosity.
|