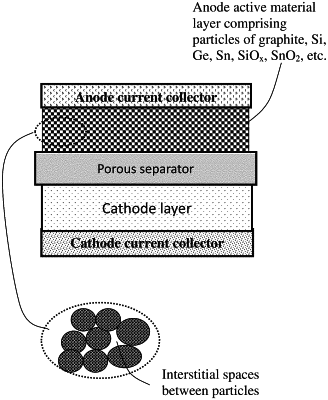
|
1. A method of improving fast-chargeability of a lithium secondary battery containing an anode, a cathode, a porous separator disposed between said anode and said cathode, and an electrolyte, wherein said method comprises packing particles of an anode active material to form an anode active material layer having interstitial spaces and disposing a lithium ion reservoir in said interstitial spaces, configured to receive lithium ions from said cathode through said porous separator when said battery is charged and to enable said lithium ions to enter said particles of anode active material in a time-delayed manner, wherein said lithium ion reservoir comprises lithium-capturing groups dispersed in a fluid residing in said interstitial spaces and said lithium-capturing groups are selected from (a) electron-donating groups interspaced between non-electron-donating groups; (b) anions and cations wherein the anions are more mobile than the cations; (c) an ionic liquid; or (d) a combination thereof, wherein said particles of anode active material are coated with molecules of poly(lithium-4-styrenesulfonate).
|