| CPC G16H 50/30 (2018.01) [A61B 5/0006 (2013.01); A61B 5/28 (2021.01); A61B 5/318 (2021.01); A61B 5/7275 (2013.01); G16H 50/20 (2018.01); G06F 18/2155 (2023.01)] | 30 Claims |
|
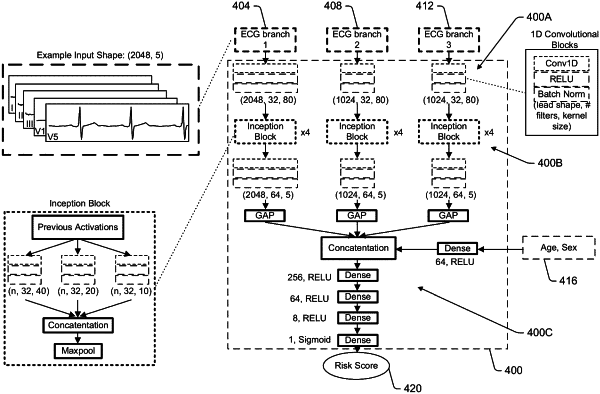
1. A method for determining cardiac amyloidosis risk from electrocardiogram trace data, interventricular septal thickness (IVSD) data, and clinical data, comprising:
receiving electrocardiogram (ECG) trace data associated with a patient, the electrocardiogram trace data having an electrocardiogram configuration including a plurality of leads and a time interval and comprising, for each lead included in the plurality of leads, voltage data associated with at least a portion of the time interval;
providing a first portion of the ECG trace data to a feature extraction layer, the first portion of the ECG trace data consisting of ECG data acquired at a body surface of the patient, the feature extraction layer being trained to predict a representation of IVSD thickness from the voltage data of the ECG trace data;
outputting, from the feature extraction layer, a representation of IVSD thickness;
receiving clinical data associated with the patient;
providing the representation of IVSD thickness, the clinical data and at least a second portion of the electrocardiogram trace data to a second layer, the second layer being a layer of a trained machine learning model, the second layer trained to evaluate the representation of IVSD thickness, the clinical data, and the second portion of the electrocardiogram trace data with respect to cardiac amyloidosis;
generating, by the trained machine learning model and based on the evaluation, a risk score reflecting a likelihood of the patient being diagnosed with cardiac amyloidosis within a predetermined period of time from when the ECG trace data was generated; and
outputting the risk score to at least one of a memory or a display.
|