| CPC G06T 7/0012 (2013.01) [A61B 5/055 (2013.01); G16B 5/00 (2019.02); G16C 20/30 (2019.02); G16H 20/00 (2018.01); G06T 2207/10088 (2013.01); G06T 2207/30101 (2013.01)] | 23 Claims |
|
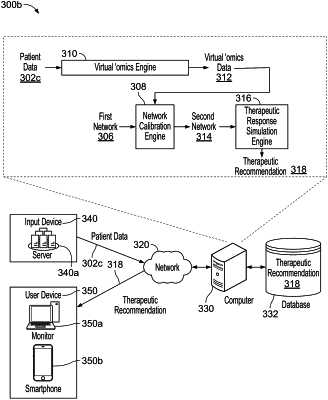
1. A method of providing a recommendation of a combination of any two or more therapies selected from a lipid-lowering therapy, an anti-inflammatory therapy, and an anti-diabetic therapy, for a patient diagnosed with atherosclerotic cardiovascular disease, the method comprising:
receiving non-invasively obtained data related to a plaque from the patient;
accessing a systems biology model of atherosclerotic cardiovascular disease, wherein
(i) the systems biology model represents a plurality of pathways associated with atherosclerotic cardiovascular disease,
(ii) the plurality of pathways include pathways corresponding, respectively, to all three of:
a) one or more of glycosylated low-density lipoprotein (glyLDL), oxidized low-density lipoprotein (oxLDL), and minimally-modified low-density lipoprotein (mmLDL), or very-low-density lipoprotein (VLDL),
b) one or more of Interleukin-1 (IL-1), Interleukin-1 beta (IL1β), Tumor Necrosis Factor (TNF), Interleukin-12/23 (IL12/23), Interleukin-17 (IL17), or other cytokine molecule, and
c) one or more of Mammalian Target of Rapamycin (MTOR), Nuclear Factor kappa B-1 (NFκβ1), Intercellular Adhesion Molecule 1 (ICAM1), or Vascular Cell Adhesion Molecule 1 (VCAM1), and
(iii) the systems biology model includes a disease-associated molecule level for each molecule in the systems biology model;
updating the systems biology model using personalized molecule levels derived from the non-invasively obtained data from the patient to generate a patient-specific systems biology model;
updating the patient-specific systems biology model with information relating to an effect on low-density lipoprotein (LDL) levels by a lipid lowering agent, inflammation levels by an anti-inflammatory agent, and glucose levels by an anti-diabetic agent, based on known mechanisms of action of each of the agents;
simulating a therapeutic response by the patient to a combination of any two or more of the lipid lowering agent, the anti-inflammatory agent, and the anti-diabetic agent in the updated patient-specific systems biology model to obtain simulated therapeutic effects for two or more combinations;
comparing the updated patient-specific systems biology model with and without the simulated therapeutic effects for each of the two or more combinations; and
based on the comparison, providing a report recommending for the patient a combination of therapeutic agents.
|