| CPC G03F 7/0757 (2013.01) [C07C 235/34 (2013.01); C07D 211/22 (2013.01); C08G 77/04 (2013.01); C09D 183/04 (2013.01); G03F 7/0045 (2013.01); G03F 7/162 (2013.01); G03F 7/168 (2013.01); G03F 7/2004 (2013.01); G03F 7/322 (2013.01); G03F 7/38 (2013.01); G03F 7/40 (2013.01)] | 17 Claims |
|
1. A negative type photosensitive siloxane composition, comprising:
(I) a polysiloxane
which contains a repeating unit represented by the following formula (Ia):
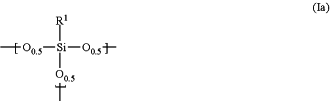 wherein
R1 is hydrogen, a monovalent to trivalent, linear, branched or cyclic, saturated or unsaturated C1-30 aliphatic hydrocarbon group, or a monovalent to trivalent C6-30 aromatic hydrocarbon group,
in said aliphatic hydrocarbon group and said aromatic hydrocarbon group, one or more methylene are unsubstituted or substituted with oxy, imide or carbonyl, one or more hydrogens are unsubstituted or substituted with fluorine, hydroxy or alkoxy, or one or more carbons are unsubstituted or substituted with silicon, when R1 is divalent or trivalent, R1 connects Si atoms contained in a plurality of repeating units; and
a repeating unit represented by the following formula (lb):
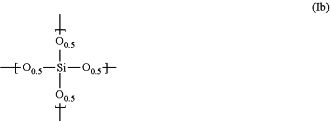 and further
which shows a spectrum in which the area intensities S1 and S2 of the peaks in the ranges of 1100±100 cm−1 and 900±100 cm−1 assigned to Si—O and SiOH, respectively, are in a S2/S1 ratio of 0.05 to 0.15 when measured and analyzed by FT-IR,
(II) a photo base generator
represented by the following formula (PBG-A) or (PBG-B):
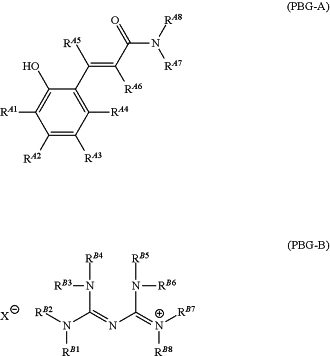 wherein
each of RA1 to RA6 is independently hydrogen, halogen, hydroxy, mercapto, sulfide, silyl, silanol, nitro, nitroso, sulfino, sulfo, sulfonate, phosphino, phosphinyl, phosphono, phosphonato, amino, ammonium, an unsubstituted or substituted C1-20 aliphatic hydrocarbon group, an unsubstituted or substituted C6-22 aromatic hydrocarbon group, unsubstituted or substituted C1-20 alkoxy, or unsubstituted or substituted C6-20 aryloxy;
each of RA7 and RA8 is independently C1-6 hydroxyalkyl;
each of RB1 to RB8 is independently hydrogen, an unsubstituted or substituted C1-20 aliphatic hydrocarbon group, an unsubstituted or substituted C6-22 aromatic hydrocarbon group, unsubstituted or substituted C1-20 alkoxy, or unsubstituted or substituted C6-20 aryloxy; and
X− is a borate or carboxylate ion having an unsubstituted or substituted C1-20 aliphatic hydrocarbon group or an unsubstituted or substituted C6-22 aromatic hydrocarbon group; and
(III) a solvent.
|