| CPC G02B 5/3016 (2013.01) [C08J 7/0427 (2020.01); C09D 7/65 (2018.01); C09D 135/02 (2013.01); C09K 19/3497 (2013.01); C09K 19/3823 (2013.01); C09K 19/3861 (2013.01); G02B 1/04 (2013.01); C08J 2400/12 (2013.01); C08J 2467/03 (2013.01); C09K 2019/0448 (2013.01); C09K 2219/03 (2013.01); C09K 2323/035 (2020.08)] | 10 Claims |
|
1. A laminate film comprising a base film; and a retardation layer formed on one side of the base film,
wherein the retardation layer has a quarter-wave phase delay characteristics, and comprises a polymerized unit of a normal wavelength dispersion polymerizable liquid crystal compound and a polymerized unit of a reverse wavelength dispersion polymerizable liquid crystal compound,
wherein the retardation layer comprises neither an ultraviolet absorber nor a light stabilizer, and
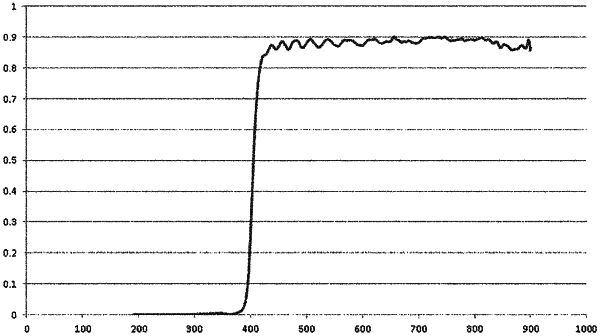
wherein the retardation layer has an ultraviolet absorptivity such that a transmittance of light having a wavelength of 385 nm through the retardation layer is 3% or less, a transmittance of light having a wavelength of 390 nm through the retardation layer is greater than 3% and less than 10%, and a transmittance of light having a wavelength of 395 nm through the retardation layer is greater than or equal to 10% and less than 25%; and
wherein the reverse wavelength dispersion polymerizable liquid crystal compound is represented by Formula 1 below:
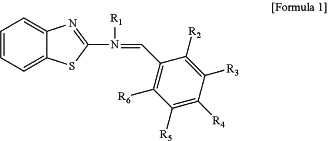 wherein in Formula 1, R1 is a substituent of Formula 2 or 3 below, and R2 to R6 are each independently hydrogen, an alkyl group, an alkoxy group, a cyano group, a substituent of Formula 4 below or a substituent of Formula 5 below, wherein at least two of R2 to R6 are substituents of Formula 4 below or substituents of Formula 5 below,
-A1-L1-Cyc-A2-L2-P [Formula 2]
wherein, in Formula 2, A1 and A2 are each independently an oxygen atom or a single bond, L1 and L2 are each independently —C(═O)—O—, —O—C(═O)— or an alkylene group, Cyc is an arylene group or a cycloalkylene group, and P is an acryloyl group, a methacryloyl group, an acryloyloxy group or a methacryloyloxy group,
 wherein, in Formula 3, L3 and L4 are each an alkylene group, n is a number in a range of 1 to 4, and P is an acryloyl group, a methacryloyl group, an acryloyloxy group, a methacryloyloxy group or a hydrogen atom,
-A3-L5-Cyc-A4-L6-P [Formula 4]
wherein in Formula 4, A3 and A4 are an oxygen atom, an alkylene group or a single bond, L5 and L6 are each independently —C(═O)—O—, —O—C(═O)— or an alkylene group, Cyc is an arylene group, and P is an acryloyl group, a methacryloyl group, an acryloyloxy group or a methacryloyloxy group,
-A5-L7-Cy1-A6-L8-Cy2-A7-L9-P [Formula 5]
wherein in Formula 5, A5, A6 and A7 are each independently an oxygen atom or a single bond, L7, L8 and L9 are each independently —C(═O)—O—, —O—C(═O)— or an alkylene group, Cy1 is a cycloalkylene group, Cy2 is an arylene group, and P is an acryloyl group, a methacryloyl group, an acryloyloxy group or a methacryloyloxy group,
wherein the reverse wavelength dispersion polymerizable liquid crystal compound includes a reverse wavelength dispersion polymerizable liquid crystal compound having tri-functionality or more; and
wherein the normal wavelength dispersion polymerizable liquid crystal compound is represented by Formula 6 below:
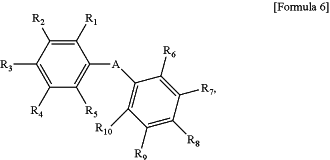 wherein in Formula 6, A is a single bond, —C(═O)O— or —OC(═O)— and R1 to R10 are each independently hydrogen, halogen, an alkyl group, an alkoxy group, an alkoxycarbonyl group, a cyano group, a nitro group or a substituent of Formual 7 below, or two neighboring substituents selected from R1 to R5, or two neighboring substituents selected from R6 to R10, are bonded to each other to constitute a benzene ring substituted with an -L′-A′-P′ group, wherein in the -L′-A′-P′ group, L′ is —C(═O)O—, —OC(═O)— or —OC(═O)O—, A′ is an alkylene group and P′ is an acryloyl group, a methacryloyl group, an acryloyloxy group or a methacryloyloxy group,
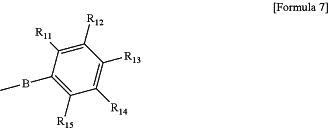 wherein in Formula 7, B is a single bond, —C(═O)O— or —OC(═O)— and R11 to R15 are each independently hydrogen, halogen, an alkyl group, an alkoxy group, an alkoxycarbonyl group, a cyano group, a nitro group or an -L-A-P group, or two neighboring substituents of R11 to R15 are bonded to each other to constitute a benzene ring substituted with an -L-A-P group, wherein in the -L-A-P group, L is —C(═O)O—, —OC(═O)— or —OC(═O)O—, A is an alkylene group and P is an acryloyl group, a methacryloyl group, an acryloyloxy group or a methacryloyloxy group.
|