| CPC G01N 33/582 (2013.01) [C07D 209/14 (2013.01); C07D 311/82 (2013.01); C07D 311/90 (2013.01); C07D 405/12 (2013.01); C07D 413/04 (2013.01); C07D 491/22 (2013.01); C07F 5/02 (2013.01); C07K 1/13 (2013.01); C09B 7/00 (2013.01); C09B 11/12 (2013.01); C09B 11/24 (2013.01); C09B 11/28 (2013.01); C09B 57/00 (2013.01); C09B 69/00 (2013.01); C09K 11/06 (2013.01); G01N 31/22 (2013.01); G01N 33/5005 (2013.01); G01N 33/5058 (2013.01); G01N 33/5091 (2013.01); G01N 33/80 (2013.01); G01N 33/84 (2013.01); G01N 2333/245 (2013.01); Y10T 436/143333 (2015.01)] | 20 Claims |
|
1. A method for detecting internalization of a carrier molecule, the method comprising:
(a) conjugating the carrier molecule to a fluorescent pH-sensitive dye of Formula I:
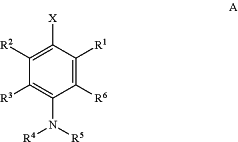 wherein,
R1, R2, R3 and R6 are each independently H, Z, or an electron donating group (EDG), provided that R1 and R2 are not hydroxyl or thiol or their deprotonated forms;
R4 is selected from the group consisting of H, alkyl, substituted alkyl, acyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, a reactive group, a carrier molecule, heterocyclyl, and substituted heterocyclyl;
R5 is independently selected from the group consisting of Y, alkyl, substituted alkyl, alkenyl, substituted alkenyl, acyl, aryl, substituted aryl, heteroaryl, substituted heteroaryl, cycloalkyl, substituted cycloalkyl, a reactive group, a carrier molecule, heterocyclyl, and substituted heterocyclyl;
X is a fluorophore;
Y is ═CRbRc or ═CRbRd;
Z is —ORc, —SRc, —NRbRc;
Rb is H, alkyl, or substituted alkyl;
Rc is alkyl or substituted alkyl; and
Rd is amino or substituted amino;
or a stereoisomer, tautomer, or salt thereof to form a carrier molecule conjugate;
(b) contacting the carrier molecule conjugate with a cell to form a contacted cell;
(c) incubating the contacted cell to form an incubated solution;
(d) illuminating the incubated solution to form an illuminated solution; and
(e) detecting fluorescent emissions from the illuminated solution;
wherein fluorescent emissions indicate internalization of the carrier molecule and wherein no quenching step is performed before the detecting step.
|