| CPC C22B 7/007 (2013.01) [C01B 33/14 (2013.01); C01B 33/26 (2013.01); C01B 33/32 (2013.01); C01F 7/0693 (2013.01); C01F 11/02 (2013.01); C01F 11/24 (2013.01); C01F 17/271 (2020.01); C01G 49/02 (2013.01); C01G 49/06 (2013.01); C01G 49/10 (2013.01); C22B 3/065 (2013.01); C22B 3/08 (2013.01); C22B 3/10 (2013.01); C22B 3/42 (2013.01); C22B 3/44 (2013.01); C22B 7/02 (2013.01); C22B 21/0023 (2013.01); C22B 59/00 (2013.01); C01D 13/00 (2013.01)] | 21 Claims |
|
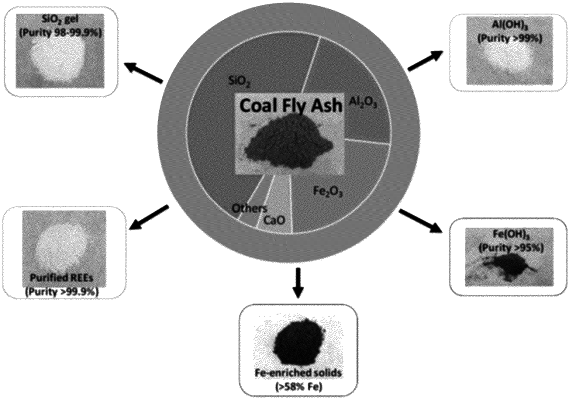
1. A process for preparing pure chemicals from coal ash, the process comprising:
a. extracting coal ash with a first caustic solution at a temperature elevated above ambient;
b. separating undissolved solid residue from said first caustic extracting solution, wherein said first caustic extracting solution is stored for later processing and said solid residue is washed with water;
c. treating the water washed solid residue with a first acid, wherein pH of the resulting acidic solution is maintained at about 3 and then the leftover solid is separated and washed with water;
d. extracting the water washed solid with a second caustic solution at a temperature elevated above ambient;
e. separating undissolved solid residue from the second caustic extracting solution and washing with water wherein said second caustic extracting solution is stored for later processing;
f. treating the undissolved solid residue with a second acid to bring the pH of the resulting acidic solution to about 3, and isolating the leftover solid as a purified iron oxide with aluminum silicate;
g. combining the acidic extraction solutions from steps c and f, and adjusting the pH to about 5 with a base whereby aluminum silicate precipitates out from the solution as a product and the leftover solution contains enriched rare earth elements (REEs); and
h. combining the first and the second caustic extracting solutions to recover purified sodium silicate by pH adjustment with ana third acid, wherein at least one respective caustic extraction solution includes sodium hydroxide and wherein at least one respective caustic extraction solution includes potassium hydroxide.
|