| CPC C12Q 1/6883 (2013.01) [C12Q 1/6865 (2013.01); G01N 33/50 (2013.01); C12Q 2600/106 (2013.01); C12Q 2600/156 (2013.01)] | 3 Claims |
|
1. A method, comprising:
obtaining a sample comprising urine from a subject who has, or is suspected to have, myotonic dystrophy type 1 (DM1);
isolating extracellular mRNA in the sample;
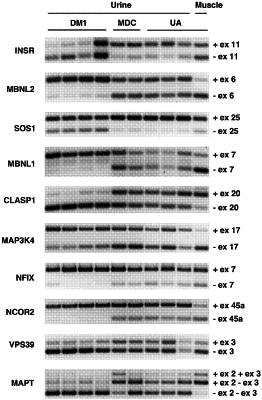
performing an assay to detect isoforms of mRNAs in the sample, wherein the mRNAs are:
(i) transcripts for muscleblind like splicing regulator 2 (MBNL2); SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS1); cytoplasmic linker associated protein 1 (CLASP1);
muscleblind like splicing regulator 1 (MBNL1); and mitogen-activated protein kinase kinase kinase 4 (MAP3K4), or
(ii) transcripts for MBNL2, MBNL1, SOS1, CLASP1, MAP3K4, and insulin receptor (INSR).
|