| CPC C12M 41/26 (2013.01) [C12M 41/36 (2013.01); G01D 18/008 (2013.01); G01N 27/4165 (2013.01); G05D 21/02 (2013.01)] | 20 Claims |
|
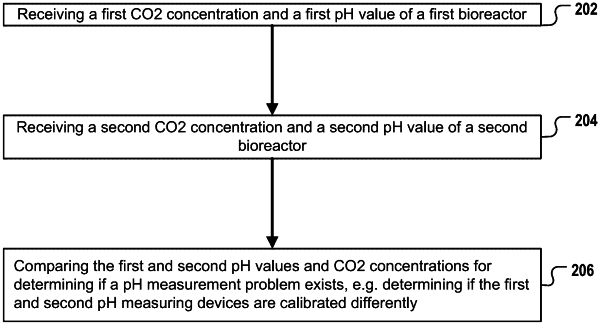
1. A method for determining if a first pH measuring device operatively coupled to a first tank is affected by a pH measuring problem, the problem being that the first pH measuring device is calibrated differently than a second pH measuring device operatively coupled to a second tank, the method comprising:
receiving, by a comparison unit, a first CO2 concentration and a first pH value, the first CO2 concentration being a CO2 concentration of a first gas volume above a medium in the first tank, the first CO2 concentration and the first pH value being measured at a first time, the first time being a time when the medium in the first tank is in pH-CO2 equilibrium state with the first gas volume at a predefined temperature and pressure, said equilibrium state being unaffected by the metabolism of any cell culture, the first pH value being a measured value provided by the first pH measuring device;
receiving, by the comparison unit, a second CO2 concentration and a second pH value, the second CO2 concentration being a CO2 concentration of a second gas volume above the same type of medium contained in the second tank, the second CO2 concentration and the second pH value being measured at a second time, the second time being a time when the medium in the second tank is in pH-CO2 equilibrium state with the second gas volume at the predefined temperature and pressure, said equilibrium state being unaffected by the metabolism of any cell culture, the second pH value being a measured value provided by the second pH measuring device;
comparing, by the comparison unit, the first and second pH values and comparing the first and second CO2 concentrations for determining that the first pH measuring device is affected by the pH measuring problem;
calibrating the first pH measuring device based on determining that the first pH measuring device is affected by the pH measuring problem; and
measuring a calibrated first pH value using the calibrated first pH measuring device.
|