| CPC C09B 69/105 (2013.01) [C09B 23/0066 (2013.01); C09B 23/083 (2013.01); C09B 23/107 (2013.01); C09K 11/06 (2013.01); C09K 2211/1466 (2013.01)] | 20 Claims |
|
1. A polymerizable near-IR dye having a structure according to formula (I):
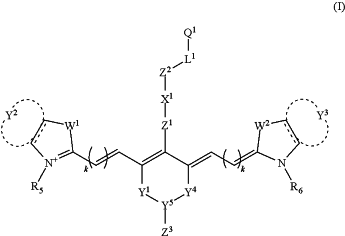 or a tautomer, salt, hydrate, or solvate thereof,
wherein:
Z1 is absent, O, NRZ1, or S;
RZ1 is H, optionally substituted C1-C10 alkyl, or optionally substituted C2-C10 heteroalkyl;
X1 is absent, optionally substituted C6-C10 arylene, or optionally substituted C1-C10 heteroarylene;
Z2 is absent, NRX1, O, S, NRX2C(O), OC(O), SC(O), C(O)O, C(O)NRX3, C(S)O, or C(O)S;
Z3 is absent or H;
RX1, RX2, and RX3 are each independently H, optionally substituted C1-C10 alkyl, or optionally substituted C1-C10 heteroalkyl;
L1 is absent, optionally substituted C1-C30 alkylene, or optionally substituted C1-C30 heteroalkylene;
Q1 is NRZ3C(O)C(RQ1)CH2, OC(O)C(RQ1)CH2, or SC(O)C(RQ1)CH2;
RZ3 is H, optionally substituted C1-C10 alkyl, optionally substituted C3-C10 cycloalkyl, optionally substituted C1-C10 heteroalkyl, or optionally substituted C1-C10 cycloheteroalkyl;
RQ1 is H, Me, Et, or n-Pr;
each of Y1 and Y4, independently, is absent or optionally substituted C1-C4 alkylene or optionally substituted C2-C4 heteroalkylene;
each of Y2 and Y3, independently, is absent or, together with the carbon atoms to which each is attached, form optionally substituted C6-C10 aryl or optionally substituted C2-C10 heteroaryl;
Y5 is absent or N or CR7;
R7 is H, optionally substituted C1-C10 alkyl, optionally substituted C3-C10 cycloalkyl, optionally substituted C1-C10 heteroalkyl, or optionally substituted C1-C10 cycloheteroalkyl;
each of W1 and W2, independently, is NR2, S, O, Se, or CR3R4;
R2, R3, and R4 are independently H, optionally substituted C1-C30 alkyl, optionally substituted C1-C30 heteroalkyl, or -L2-Q2;
L2 is optionally substituted C1-C30 alkylene or optionally substituted C1-C30 heteroalkylene;
Q2 is H, C(RQ1)CH2, C6H4CHCH2, NRZ3C(O)C(RQ1)CH2, OC(O)C(RQ1)CH2, or SC(O)C(RQ1)CH2;
each of R5 and R6, independently, is -L3-Q3, optionally substituted C1-C30 alkyl, or optionally substituted C6-C10 aryl;
L3 is optionally substituted C1-C30 alkylene or optionally substituted C1-C30 heteroalkylene;
Q3 is H, C(RQ1)CH2, C6H4CHCH2, NRZ3C(O)C(RQ1)CH2, OC(O)C(RQ1)CH2, or SC(O)C(RQ1)CH2; and
each k is independently 0 or 1.
|